IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis
- PMID: 15286809
- PMCID: PMC484976
- DOI: 10.1172/JCI20479
IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis
Abstract
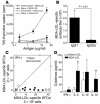
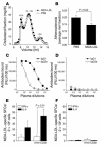
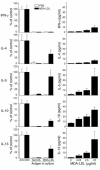
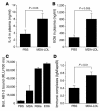
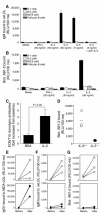
During atherogenesis, LDL is oxidized, generating various oxidation-specific neoepitopes, such as malondialdehyde-modified (MDA-modified) LDL (MDA-LDL) or the phosphorylcholine (PC) headgroup of oxidized phospholipids (OxPLs). These epitopes are recognized by both adaptive T cell-dependent (TD) and innate T cell-independent type 2 (TI-2) immune responses. We previously showed that immunization of mice with MDA-LDL induces a TD response and atheroprotection. In addition, a PC-based immunization strategy that leads to a TI-2 expansion of innate B-1 cells and secretion of T15/EO6 clonotype natural IgM antibodies, which bind the PC of OxPLs within oxidized LDL (OxLDL), also reduces atherogenesis. T15/EO6 antibodies inhibit OxLDL uptake by macrophages. We now report that immunization with MDA-LDL, which does not contain OxPL, unexpectedly led to the expansion of T15/EO6 antibodies. MDA-LDL immunization caused a preferential expansion of MDA-LDL-specific Th2 cells that prominently secreted IL-5. In turn, IL-5 provided noncognate stimulation to innate B-1 cells, leading to increased secretion of T15/EO6 IgM. Using a bone marrow transplant model, we also demonstrated that IL-5 deficiency led to decreased titers of T15/EO6 and accelerated atherosclerosis. Thus, IL-5 links adaptive and natural immunity specific to epitopes of OxLDL and protects from atherosclerosis, in part by stimulating the expansion of atheroprotective natural IgM specific for OxLDL.
Figures
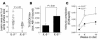
Comment in
-
IL-5 links adaptive and natural immunity in reducing atherosclerotic disease.J Clin Invest. 2004 Aug;114(3):317-9. doi: 10.1172/JCI22561. J Clin Invest. 2004. PMID: 15286796 Free PMC article. Review.
References
-
- Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat. Med. 2002;8:1211–1217. - PubMed
-
- Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. - PubMed
-
- Binder CJ, et al. Innate and acquired immunity in atherogenesis. Nat. Med. 2002;8:1218–1226. - PubMed
-
- Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002;91:281–291. - PubMed
-
- Hörkkö S, et al. Immunological responses to oxidized LDL. Free Radic. Biol. Med. 2000;28:1771–1779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

