Identification of proteases employed by dendritic cells in the processing of protein purified derivative (PPD)
- PMID: 15287985
- PMCID: PMC514720
- DOI: 10.1186/1476-8518-2-8
Identification of proteases employed by dendritic cells in the processing of protein purified derivative (PPD)
Abstract
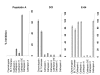
Dendritic cells (DC) are known to present exogenous protein Ag effectively to T cells. In this study we sought to identify the proteases that DC employ during antigen processing. The murine epidermal-derived DC line Xs52, when pulsed with PPD, optimally activated the PPD-reactive Th1 clone LNC.2F1 as well as the Th2 clone LNC.4k1, and this activation was completely blocked by chloroquine pretreatment. These results validate the capacity of XS52 DC to digest PPD into immunogenic peptides inducing antigen specific T cell immune responses. XS52 DC, as well as splenic DC and DCs derived from bone marrow degraded standard substrates for cathepsins B, C, D/E, H, J, and L, tryptase, and chymases, indicating that DC express a variety of protease activities. Treatment of XS52 DC with pepstatin A, an inhibitor of aspartic acid proteases, completely abrogated their capacity to present native PPD, but not trypsin-digested PPD fragments to Th1 and Th2 cell clones. Pepstatin A also inhibited cathepsin D/E activity selectively among the XS52 DC-associated protease activities. On the other hand, inhibitors of serine proteases (dichloroisocoumarin, DCI) or of cystein proteases (E-64) did not impair XS52 DC presentation of PPD, nor did they inhibit cathepsin D/E activity. Finally, all tested DC populations (XS52 DC, splenic DC, and bone marrow-derived DC) constitutively expressed cathepsin D mRNA. These results suggest that DC primarily employ cathepsin D (and perhaps E) to digest PPD into antigenic peptides.
Figures




Similar articles
-
T cell-dependent loss of proliferative responsiveness to colony-stimulating factor-1 by a murine epidermal-derived dendritic cell line, XS52.J Immunol. 1995 Dec 1;155(11):5190-7. J Immunol. 1995. PMID: 7594529
-
Ultraviolet B radiation sensitizes a murine epidermal dendritic cell line (XS52) to undergo apoptosis upon antigen presentation to T cells.J Immunol. 1996 Oct 15;157(8):3312-6. J Immunol. 1996. PMID: 8871626
-
Cytokine-mediated communication between dendritic epidermal T cells and Langerhans cells. In vitro studies using cell lines.J Immunol. 1996 Aug 15;157(4):1529-37. J Immunol. 1996. PMID: 8759735
-
T cell-dependent secretion of IL-1 beta by a dendritic cell line (XS52) derived from murine epidermis.J Immunol. 1995 Oct 15;155(8):3794-800. J Immunol. 1995. PMID: 7561084
-
Proteases involved in MHC class II antigen presentation.Immunol Rev. 1999 Dec;172:109-20. doi: 10.1111/j.1600-065x.1999.tb01360.x. Immunol Rev. 1999. PMID: 10631941 Review.
Cited by
-
Procathepsin D secreted by HaCaT keratinocyte cells - A novel regulator of keratinocyte growth.Eur J Cell Biol. 2007 Jun;86(6):303-13. doi: 10.1016/j.ejcb.2007.03.008. Eur J Cell Biol. 2007. PMID: 17532541 Free PMC article.
-
Immunomodulatory role of proteinase-activated receptor-2.Ann Rheum Dis. 2012 Sep;71(9):1559-66. doi: 10.1136/annrheumdis-2011-200869. Epub 2012 May 6. Ann Rheum Dis. 2012. PMID: 22563031 Free PMC article.
-
Cathepsin S Is Involved in Th17 Differentiation Through the Upregulation of IL-6 by Activating PAR-2 after Systemic Exposure to Lipopolysaccharide from Porphyromonas gingivalis.Front Pharmacol. 2017 Jul 17;8:470. doi: 10.3389/fphar.2017.00470. eCollection 2017. Front Pharmacol. 2017. PMID: 28769800 Free PMC article.
-
Implications of ER stress, the unfolded protein response, and pro- and anti-apoptotic protein fingerprints in human monocyte-derived dendritic cells treated with alcohol.Alcohol Clin Exp Res. 2010 Dec;34(12):2081-8. doi: 10.1111/j.1530-0277.2010.01304.x. Epub 2010 Sep 22. Alcohol Clin Exp Res. 2010. PMID: 20860616 Free PMC article.
References
-
- Stössel H, Koch F, Kämpgen E, Stoger P, Lenz A, Heufler C, Romani N, Schuler G. Disappearance of certain acidic organelles (endosomes and Langerhans cell granules) accompanies loss of antigen processing capacity upon culture of epidermal Langerhans cells. J Exp Med. 1990;172:1471–1479. doi: 10.1084/jem.172.5.1471. - DOI - PMC - PubMed
-
- Pure E, Inaba K, Crowley M, Tardelli L, Witmer-Pack M, Ruberti G, Fathman G, Steinman R. Antigen processing by epidermal Langerhans cells correlates with the level of biosynthesis of major histocompatibility complex class II molecules and expression of invariant chain. J Exp Med. 1990;172:1459–1465. doi: 10.1084/jem.172.5.1459. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

