A randomized, controlled trial of triple antiviral therapy as initial treatment of chronic hepatitis C in HIV-infected patients
- PMID: 15288482
- PMCID: PMC7126192
- DOI: 10.1016/j.jhep.2004.04.016
A randomized, controlled trial of triple antiviral therapy as initial treatment of chronic hepatitis C in HIV-infected patients
Abstract
Background/aims: Interferon and ribavirin combination therapy for chronic hepatitis C induces a low response rate in human immunodeficiency virus (HIV) infected patients. To assess the impact of intensification of interferon administration and of the addition of amantadine on the efficacy and safety of standard anti-hepatitis C virus (HCV) treatment in HIV-infected patients.
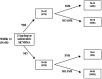
Methods: Multicentre, prospective, open-label, randomized, phase III clinical trial. Eighty co-infected patients were randomized to receive ribavirin 800-1,000 mg/day in combination with, group A: interferon alpha 2a 3MIU thrice weekly; group B: IFN alpha 2a 3MIU daily, plus amantadine 200 mg/day; treatment duration was 24-48 weeks according to HCV genotype.
Results: Forty-one patients were randomized in group A and 39 in group B. Intention-to-treat analysis showed a sustained virological response, defined as HCV-RNA negativization, 6 months after stopping treatment in 22% of patients from group A and 13% from group B (P>0.05). The lack of a 2-log drop in HCV-RNA levels after 12 weeks of treatment showed a 100% predictive value of lack of sustained response.
Conclusions: Amantadine addition and interferon intensification do not improve the low efficacy of combination of interferon alfa plus ribavirin in HIV/HCV co-infected patients. Patients with no early virologic response did not have any probability of sustained response.
Figures
References
-
- Puoti M., Spinetti A., Ghezzi A., Donato F., Zaltron S., Putzolu V. Mortality for liver disease in patients with HIV infection: a cohort study. J AIDS. 2000;24:211–217. - PubMed
-
- Soriano V., Martin-Carbonero L., Garcia-Samaniego J., Puoti M. Mortality due to chronic viral liver disease among patients with human immunodeficiency virus. Clin Infect Dis. 2001;33:1793–1794. - PubMed
-
- Poynard T., Marcellin P., Lee S., Niederau C., Minuk G.S., Ideo G. Randomised trial of interferon α2b plu ribavirin for 48 weeks or for 24 weeks vs. interferon α2b plus placebo for 48 weeks for treatment of chronic hepatitis C virus. Lancet. 1998;352:1426–1432. - PubMed
-
- Nasti G., Di Gennaro G., Tavio M., Cadorin L., Tedeschi R.M., Talamini R. Chronic hepatitis C in HIV infection: feasibility and sustained efficacy of therapy with interferon alfa-2b and ribavirin. AIDS. 2001;15:1783–1787. - PubMed
-
- Landau A., Batisse D., Piketty C., Duong Van Huyen J.P., Bloch F., Belec L. Long term efficacy of combination therapy with interferon alfa 2b and ribavirin for severe chronic hepatitis C in HIV infected patients. AIDS. 2001;15:2149–2155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

