Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by beta1 integrins
- PMID: 15289498
- PMCID: PMC2172270
- DOI: 10.1083/jcb.200403003
Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by beta1 integrins
Abstract
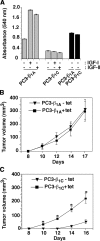
We show here that beta1 integrins selectively modulate insulin-like growth factor type I receptor (IGF-IR) signaling in response to IGF stimulation. The beta1A integrin forms a complex with the IGF-IR and insulin receptor substrate-1 (IRS-1); this complex does not promote IGF-I mediated cell adhesion to laminin (LN), although it does support IGF-mediated cell proliferation. In contrast, beta1C, an integrin cytoplasmic variant, increases cell adhesion to LN in response to IGF-I and its down-regulation by a ribozyme prevents IGF-mediated adhesion to LN. Moreover, beta1C completely prevents IGF-mediated cell proliferation and tumor growth by inhibiting IGF-IR auto-phosphorylation in response to IGF-I stimulation. Evidence is provided that the beta1 cytodomain plays an important role in mediating beta1 integrin association with either IRS-1 or Grb2-associated binder1 (Gab1)/SH2-containing protein-tyrosine phosphate 2 (Shp2), downstream effectors of IGF-IR: specifically, beta1A associates with IRS-1 and beta1C with Gab1/Shp2. This study unravels a novel mechanism mediated by the integrin cytoplasmic domain that differentially regulates cell adhesion to LN and cell proliferation in response to IGF.
Figures









Similar articles
-
beta1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts.Cancer Res. 2005 Aug 1;65(15):6692-700. doi: 10.1158/0008-5472.CAN-04-4315. Cancer Res. 2005. PMID: 16061650
-
Beta1 integrins modulate cell adhesion by regulating insulin-like growth factor-II levels in the microenvironment.Cancer Res. 2006 Jan 1;66(1):331-42. doi: 10.1158/0008-5472.CAN-05-2588. Cancer Res. 2006. PMID: 16397247
-
Tamoxifen interferes with the insulin-like growth factor I receptor (IGF-IR) signaling pathway in breast cancer cells.Cancer Res. 1997 Jul 1;57(13):2606-10. Cancer Res. 1997. PMID: 9205064
-
Type I insulin-like growth factor receptor signaling in hematological malignancies.Oncotarget. 2017 Jan 3;8(1):1814-1844. doi: 10.18632/oncotarget.12123. Oncotarget. 2017. PMID: 27661006 Free PMC article. Review.
-
Crosstalk between insulin-like growth factor (IGF) receptor and integrins through direct integrin binding to IGF1.Cytokine Growth Factor Rev. 2017 Apr;34:67-72. doi: 10.1016/j.cytogfr.2017.01.003. Epub 2017 Feb 3. Cytokine Growth Factor Rev. 2017. PMID: 28190785 Free PMC article. Review.
Cited by
-
Insulin-like growth factor I controls a mutually exclusive association of RACK1 with protein phosphatase 2A and beta1 integrin to promote cell migration.Mol Cell Biol. 2006 Jun;26(11):4041-51. doi: 10.1128/MCB.01868-05. Mol Cell Biol. 2006. PMID: 16705158 Free PMC article.
-
Nuclear localization of the ERK MAP kinase mediated by Drosophila alphaPS2betaPS integrin and importin-7.Mol Biol Cell. 2007 Oct;18(10):4190-9. doi: 10.1091/mbc.e06-07-0659. Epub 2007 Aug 15. Mol Biol Cell. 2007. PMID: 17699602 Free PMC article.
-
Extracellular influences on tumour angiogenesis in the aged host.Br J Cancer. 2008 Jan 29;98(2):250-5. doi: 10.1038/sj.bjc.6604144. Epub 2008 Jan 8. Br J Cancer. 2008. PMID: 18182993 Free PMC article. Review.
-
The anti-angiogenic peptide, loop 6, binds insulin-like growth factor-1 receptor.J Biol Chem. 2010 Dec 31;285(53):41886-95. doi: 10.1074/jbc.M110.166439. Epub 2010 Oct 12. J Biol Chem. 2010. PMID: 20940305 Free PMC article.
-
β1-integrin controls IGF-1R internalization and intracellular signaling.J Biol Chem. 2025 Jan;301(1):108021. doi: 10.1016/j.jbc.2024.108021. Epub 2024 Nov 27. J Biol Chem. 2025. PMID: 39608716 Free PMC article.
References
-
- Baserga, R. 2000. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 19:5574–5581. - PubMed
-
- Bello-DeOcampo, D., H.K. Kleinman, N.D. Deocampo, and M.M. Webber. 2001. Laminin-1 and α6β1 integrin regulate acinar morphogenesis of normal and malignant human prostate epithelial cells. Prostate. 46:142–153. - PubMed
-
- Bouvard, D., C. Brakebusch, E. Gustafsson, A. Aszodi, T. Bengtsson, A. Berna, and R. Fassler. 2001. Functional consequences of integrin gene mutations in mice. Circ. Res. 89:211–223. - PubMed
-
- Brar, P.K., B.L. Dalkin, C. Weyer, K. Sallam, I. Virtanen, and R.B. Nagle. 2003. Laminin α-1, α-3, and α-5 chain expression in human prepubertal benign prostate glands and adult benign and malignant prostate glands. Prostate. 55:65–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

