Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis
- PMID: 15289499
- PMCID: PMC2172256
- DOI: 10.1083/jcb.200312074
Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis
Abstract
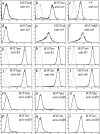
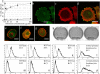
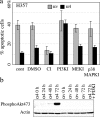
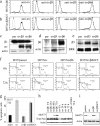
Stratified squamous epithelia express the alphavbeta5 integrin, but in squamous cell carcinomas (SCCs) there is down-regulation of alphavbeta5 and up-regulation of alphavbeta6. To investigate the significance of this finding, we transduced an alphav-negative human SCC line with retroviral vectors encoding alphav integrins. alphavbeta5-expressing cells underwent suspension-induced apoptosis (anoikis), whereas alphav-negative cells and cells expressing alphavbeta6 did not. Resistance to anoikis correlated with PKB/Akt activation in suspension, but not with changes in PTEN or p110alpha PI3 kinase levels. Anoikis was induced in parental and alphavbeta6-expressing cells by inhibiting PI3 kinase. Conversely, activation of Akt or inhibition of caspases in alphavbeta5-expressing cells suppressed anoikis. Caspase inhibition resulted in increased phosphoAkt, placing caspase activation upstream of decreased Akt activation. Anoikis required the cytoplasmic domain of beta5 and was independent of the death receptor pathway. These results suggest that down-regulation of alphavbeta5 through up-regulation of alphavbeta6 may protect SCCs from anoikis by activating an Akt survival signal.
Figures



Similar articles
-
alphavbeta5/beta6 integrin suppression leads to a stimulation of alpha2beta1 dependent cell migration resistant to PI3K/Akt inhibition.Exp Cell Res. 2009 Jul 1;315(11):1840-9. doi: 10.1016/j.yexcr.2009.03.014. Epub 2009 Mar 26. Exp Cell Res. 2009. PMID: 19328197
-
Integrins alpha5beta1, alphavbeta1, and alphavbeta6 collaborate in squamous carcinoma cell spreading and migration on fibronectin.Exp Cell Res. 2000 Feb 25;255(1):10-7. doi: 10.1006/excr.1999.4769. Exp Cell Res. 2000. PMID: 10666329
-
Cell surface overexpression of alphavbeta5 integrin impedes alphavbeta3-mediated migration of the human ovarian adenocarcinoma cell line IGROV1.Cell Biol Int. 2007 Feb;31(2):109-18. doi: 10.1016/j.cellbi.2006.09.013. Epub 2006 Sep 23. Cell Biol Int. 2007. PMID: 17074516
-
Alphavbeta6 integrin in wound healing and cancer of the oral cavity.J Oral Pathol Med. 2006 Jan;35(1):1-10. doi: 10.1111/j.1600-0714.2005.00374.x. J Oral Pathol Med. 2006. PMID: 16393247 Review.
-
Anoikis mediators in oral squamous cell carcinoma.Oral Dis. 2011 May;17(4):355-61. doi: 10.1111/j.1601-0825.2010.01763.x. Epub 2010 Nov 29. Oral Dis. 2011. PMID: 21114588 Free PMC article. Review.
Cited by
-
Expression of Integrin β6 and HAX-1 Correlates with Aggressive Features and Poor Prognosis in Esophageal Squamous Cell Carcinoma.Cancer Manag Res. 2020 Oct 2;12:9599-9608. doi: 10.2147/CMAR.S274892. eCollection 2020. Cancer Manag Res. 2020. PMID: 33061645 Free PMC article.
-
Understanding micrometastatic disease and Anoikis resistance in ewing family of tumors and osteosarcoma.Oncologist. 2010;15(6):627-35. doi: 10.1634/theoncologist.2010-0093. Epub 2010 May 17. Oncologist. 2010. PMID: 20479280 Free PMC article. Review.
-
Differences in integrin expression and signaling within human breast cancer cells.BMC Cancer. 2011 Jul 13;11:293. doi: 10.1186/1471-2407-11-293. BMC Cancer. 2011. PMID: 21752268 Free PMC article.
-
Integrin Regulation of Epidermal Functions in Wounds.Adv Wound Care (New Rochelle). 2014 Mar 1;3(3):229-246. doi: 10.1089/wound.2013.0516. Adv Wound Care (New Rochelle). 2014. PMID: 24669359 Free PMC article. Review.
-
Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer.BMC Cancer. 2014 Mar 1;14:145. doi: 10.1186/1471-2407-14-145. BMC Cancer. 2014. PMID: 24581231 Free PMC article.
References
-
- Agrez, M., X. Gu, J. Turton, C. Meldrum, J. Niu, T. Antalis, and E.W. Howard. 1999. The αvβ6 integrin induces gelatinase B secretion in colon cancer cells. Int. J. Cancer. 81:90–97. - PubMed
-
- Ahmed, N., J. Niu, D.J. Dorahy, X. Gu, S. Andrews, C.J. Meldrum, R.J. Scott, M.S. Baker, I.G. Macreadie, and M.V. Agrez. 2002. Direct integrin αvβ6-ERK binding: implications for tumour growth. Oncogene. 21:1370–1380. - PubMed
-
- Allombert-Blaise, C., S. Tamiji, L. Mortier, H. Fauvel, M. Tual, E. Delaporte, F. Piette, E.M. DeLassale, P. Formstecher, P. Marchetti, and R. Polakowska. 2003. Terminal differentiation of human epidermal keratinocytes involves mitochondria- and caspase-dependent cell death pathway. Cell Death Differ. 10:850–852. - PubMed
-
- Bachelder, R.E., M.A. Wendt, N. Fujita, T. Tsuruo, and A.M. Mercurio. 2001. The cleavage of Akt/protein kinase B by death receptor signaling is an important event in detachment-induced apoptosis. J. Biol. Chem. 276:34702–34707. - PubMed
-
- Breuss, J.M., J. Gallo, H.M. DeLisser, I.V. Klimanskaya, H.G. Folkesson, J.F. Pittet, S.L. Nishimura, K. Aldape, D.V. Landers, W. Carpenter, et al. 1995. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 108:2241–2251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

