Dose-dependent response of FGF-2 for lymphangiogenesis
- PMID: 15289610
- PMCID: PMC511009
- DOI: 10.1073/pnas.0404272101
Dose-dependent response of FGF-2 for lymphangiogenesis
Abstract
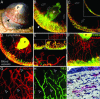
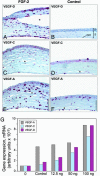
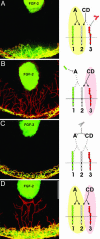
Spatio-temporal studies on the growth of capillary blood vessels and capillary lymphatic vessels in tissue remodeling have suggested that lymphangiogenesis is angiogenesis-dependent. We revisited this concept by using fibroblast growth factor 2 (FGF-2) (80 ng) to stimulate the growth of both vessel types in the mouse cornea. When we lowered the dose of FGF-2 in the cornea 6.4-fold (12.5 ng), the primary response was lymphangiogenic. Further investigation revealed that vascular endothelial growth factor-C and -D are required for this apparent lymphangiogenic property of FGF-2, and when the small amount of accompanying angiogenesis was completely suppressed, lymphangiogenesis remained unaffected. Our findings demonstrate that there is a dose-dependent response of FGF-2 for lymphangiogenesis, and lymphangiogenesis can occur in the absence of a preexisting or developing vascular bed, i.e., in the absence of angiogenesis, in the mouse cornea.
Figures

Similar articles
-
Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation.Biochem Biophys Res Commun. 2008 Dec 19;377(3):826-31. doi: 10.1016/j.bbrc.2008.10.077. Epub 2008 Oct 23. Biochem Biophys Res Commun. 2008. PMID: 18951870
-
Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability.Circ Res. 2004 Mar 19;94(5):664-70. doi: 10.1161/01.RES.0000118600.91698.BB. Epub 2004 Jan 22. Circ Res. 2004. PMID: 14739162
-
b-FGF induces corneal blood and lymphatic vessel growth in a spatially distinct pattern.Cornea. 2012 Jul;31(7):804-9. doi: 10.1097/ICO.0b013e31823f8b5a. Cornea. 2012. PMID: 22467003 Free PMC article.
-
Lymphangiogenesis Guidance Mechanisms and Therapeutic Implications in Pathological States of the Cornea.Cells. 2023 Jan 14;12(2):319. doi: 10.3390/cells12020319. Cells. 2023. PMID: 36672254 Free PMC article. Review.
-
Corneal (lymph)angiogenesis--from bedside to bench and back: a tribute to Judah Folkman.Lymphat Res Biol. 2008;6(3-4):191-201. doi: 10.1089/lrb.2008.6348. Lymphat Res Biol. 2008. PMID: 19093792 Review.
Cited by
-
Review of treatment strategies after lymphadenectomy: From molecular therapeutics to immediate microsurgical lymphatic reconstruction.J Vasc Surg Venous Lymphat Disord. 2024 Sep;12(5):101844. doi: 10.1016/j.jvsv.2024.101844. Epub 2024 Feb 3. J Vasc Surg Venous Lymphat Disord. 2024. PMID: 38316291 Free PMC article. Review.
-
Lymphatic microvessel density as a prognostic factor in non-small cell lung carcinoma: a meta-analysis of the literature.Mol Biol Rep. 2012 May;39(5):5331-8. doi: 10.1007/s11033-011-1332-y. Epub 2011 Dec 14. Mol Biol Rep. 2012. PMID: 22167333
-
Broad targeting of angiogenesis for cancer prevention and therapy.Semin Cancer Biol. 2015 Dec;35 Suppl(Suppl):S224-S243. doi: 10.1016/j.semcancer.2015.01.001. Epub 2015 Jan 16. Semin Cancer Biol. 2015. PMID: 25600295 Free PMC article. Review.
-
Endostatin specifically targets both tumor blood vessels and lymphatic vessels.Front Med. 2011 Dec;5(4):336-40. doi: 10.1007/s11684-011-0163-5. Epub 2011 Dec 27. Front Med. 2011. PMID: 22198745
-
Differential distribution of blood and lymphatic vessels in the murine cornea.Invest Ophthalmol Vis Sci. 2010 May;51(5):2436-40. doi: 10.1167/iovs.09-4505. Epub 2009 Dec 17. Invest Ophthalmol Vis Sci. 2010. PMID: 20019372 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

