Coactivator AIB1 links estrogen receptor transcriptional activity and stability
- PMID: 15289619
- PMCID: PMC511007
- DOI: 10.1073/pnas.0402997101
Coactivator AIB1 links estrogen receptor transcriptional activity and stability
Abstract
Agonist-mediated degradation of estrogen receptor alpha (ERalpha) has been associated with its transcriptional activity. However, the mechanism by which ERalpha is targeted for degradation and whether there is a direct functional link between ERalpha stability and ERalpha-mediated transactivation have not been elucidated. Here we provide evidence that the p160 coactivator, AIB1, uniquely mediates agonist-induced, but not antagonist-induced, ERalpha degradation. We show that AIB1 recruitment by ERalpha is not only necessary but also sufficient to promote degradation. Suppression of AIB1 levels leads to ERalpha stabilization in the presence of 17beta-estradiol and, despite increased ERalpha levels, reduced recruitment of ERalpha to endogenous target gene promoters. In addition, association of RNA polymerase II with ERalpha target promoters is lost when AIB1 is suppressed, leading to inhibition of target gene transcription. AIB1 thus plays a dual role in regulating ERalpha activity, one in recruiting transcription factors including other coactivators involved in gene activation and the other in regulating ERalpha protein degradation mediated by the ubiquitin-proteosome machinery.
Figures
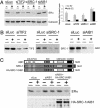

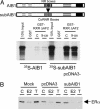
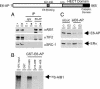

References
-
- Green, S., Walter, P., Greene, G., Krust, A., Goffin, C., Jensen, E., Scrace, G., Waterfield, M. & Chambon, P. (1986) J. Steroid Biochem. 24, 77–83. - PubMed
-
- Hall, J. M., Couse, J. F. & Korach, K. S. (2001) J. Biol. Chem. 276, 36869–36872. - PubMed
-
- Rosenfeld, M. G. & Glass, C. K. (2001) J. Biol. Chem. 276, 36865–36868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

