Elucidating the significance of beta-hydride elimination and the dynamic role of acid/base chemistry in a palladium-catalyzed aerobic oxidation of alcohols
- PMID: 15291576
- PMCID: PMC2720309
- DOI: 10.1021/ja047794s
Elucidating the significance of beta-hydride elimination and the dynamic role of acid/base chemistry in a palladium-catalyzed aerobic oxidation of alcohols
Abstract
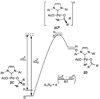
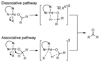
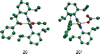
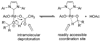
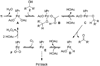
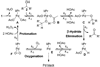
The mechanistic details of aerobic alcohol oxidation with catalytic Pd(IiPr)(OAc)(2)(H(2)O) (IiPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) are disclosed. Under optimal conditions, beta-hydride elimination is rate-limiting supported by kinetic studies including a high primary kinetic isotope effect (KIE) value of 5.5 +/- 0.1 and a Hammett rho value of -0.48 +/- 0.04. On the basis of these studies, a late transition state is proposed for beta-hydride elimination, which is further corroborated by theoretical calculations using density functional theory. Additive acetic acid modulates the rates of both the alcohol oxidation sequence and regeneration of the Pd catalyst. With no additive [HOAc], turnover-limiting reprotonation of intermediate palladium peroxo is kinetically competitive with beta-hydride elimination, allowing for reversible oxygenation and decomposition of Pd(0). With additive [HOAc] (>2 mol %), reprotonation of the palladium peroxo is fast and beta-hydride elimination is the single rate-controlling step. This proposal is supported by an apparent decomposition pathway modulated by [HOAc], a change in alcohol concentration dependence, a lack of [O(2)] dependence at high [HOAc], and significant changes in the KIE values at different HOAc concentrations.
Figures
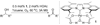













References
-
- Berzelius J. J. Ann. Phys. Chem. 1828;13:435–488.
-
- Moreno-Manas M, Pleixats R, Villarroya S. Organometallics. 2001;20:4524–4528.
- Bellosta V, Benhaddou R, Czernecki S. Synlett. 1993:861–863.
- Benhaddou R, Czernecki S, Ville G, Zegar A. Organometallics. 1988;7:2435–2439.
- Zaw K, Lautens M, Henry PM. Organometallics. 1983;2:197–199.
- Tomioka H, Takai K, Oshina K, Nozaki H. Tetrahedron Lett. 1981;22:1605–1608.
-
-
For a recent review on Pd-catalyzed oxidations of alcohols, see: Muzart J. Tetrahedron. 2003;59:5789–5816.
-
-
- Nikiforova AV, Moiseev II, Syrkin YK. Zh. Obshch. Khim. 1964;33:3239–3242.
-
- Lloyd WG. J. Org. Chem. 1967;32:2816–2819.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

