Isoleucine biosynthesis in Leptospira interrogans serotype lai strain 56601 proceeds via a threonine-independent pathway
- PMID: 15292141
- PMCID: PMC490871
- DOI: 10.1128/JB.186.16.5400-5409.2004
Isoleucine biosynthesis in Leptospira interrogans serotype lai strain 56601 proceeds via a threonine-independent pathway
Abstract
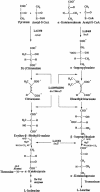
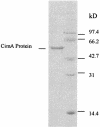
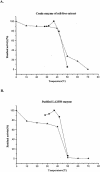
Three leuA-like protein-coding sequences were identified in Leptospira interrogans. One of these, the cimA gene, was shown to encode citramalate synthase (EC 4.1.3.-). The other two encoded alpha-isopropylmalate synthase (EC 4.1.3.12). Expressed in Escherichia coli, the citramalate synthase was purified and characterized. Although its activity was relatively low, it was strictly specific for pyruvate as the keto acid substrate. Unlike the citramalate synthase of the thermophile Methanococcus jannaschii, the L. interrogans enzyme is temperature sensitive but exhibits a much lower K(m) (0.04 mM) for pyruvate. The reaction product was characterized as (R)-citramalate, and the proposed beta-methyl-d-malate pathway was further confirmed by demonstrating that citraconate was the substrate for the following reaction. This alternative pathway for isoleucine biosynthesis from pyruvate was analyzed both in vitro by assays of leptospiral isopropylmalate isomerase (EC 4.2.1.33) and beta-isopropylmalate dehydrogenase (EC 1.1.1.85) in E. coli extracts bearing the corresponding clones and in vivo by complementation of E. coli ilvA, leuC/D, and leuB mutants. Thus, the existence of a leucine-like pathway for isoleucine biosynthesis in L. interrogans under physiological conditions was unequivocally proven. Significant variations in either the enzymatic activities or mRNA levels of the cimA and leuA genes were detected in L. interrogans grown on minimal medium supplemented with different levels of the corresponding amino acids or in cells grown on serum-containing rich medium. The similarity of this metabolic pathway in leptospires and archaea is consistent with the evolutionarily primitive status of the eubacterial spirochetes.
Figures


References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. A. Smith, and K. Struhl. 1991. Molecular cloning: a laboratory manual. Greene and Wiley Interscience, New York, N.Y.
-
- Bauby, H., I. Saint Girons, and M. Picardeau. 2003. Construction and complementation of the first auxotrophic mutant in the spirochaete Leptospira meyeri. Microbiology 149(Pt. 3):689-693. - PubMed
-
- Belly, R. T., S. Greenfield, and G. W. Claus. 1970. β-Methylaspartate metabolism and isoleucine biosynthesis in Acetobacter suboxydans. Bacteriol. Proc., p. 141.
-
- Blatt, J. M., W. J. Pledger, and H. E. Umbarger. 1972. Isoleucine and valine metabolism in Escherichia coli. XX. Multiple forms of acetohydroxy acid synthetase. Biochem. Biophys. Res. Commun. 48:444-450. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

