Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter
- PMID: 15292151
- PMCID: PMC490934
- DOI: 10.1128/JB.186.16.5486-5495.2004
Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter
Abstract
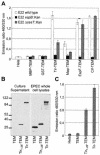
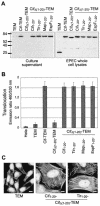
Enteropathogenic and enterohemorrhagic Escherichia coli (EPEC and EHEC) strains are human and animal pathogens that inject effector proteins into host cells via a type III secretion system (TTSS). Cif is an effector protein which induces host cell cycle arrest and reorganization of the actin cytoskeleton. Cif is encoded by a lambdoid prophage present in most of the EPEC and EHEC strains. In this study, we analyzed the domain that targets Cif to the TTSS by using a new reporter system based on a translational fusion of the effector proteins with mature TEM-1 beta-lactamase. Translocation was detected directly in living host cells by using the fluorescent beta-lactamase substrate CCF2/AM. We show that the first 16 amino acids (aa) of Cif were necessary and sufficient to mediate translocation into the host cells. Similarly, the first 20 aa of the effector proteins Map, EspF, and Tir, which are encoded in the same region as the TTSS, mediated secretion and translocation in a type III-dependent but chaperone-independent manner. A truncated form of Cif lacking its first 20 aa was no longer secreted and translocated, but fusion with the first 20 aa of Tir, Map, or EspF restored both secretion and translocation. In addition, the chimeric proteins were fully able to trigger host cell cycle arrest and stress fiber formation. In conclusion, our results demonstrate that Cif is composed of a C-terminal effector domain and an exchangeable N-terminal translocation signal and that the TEM-1 reporter system is a convenient tool for the study of the translocation of toxins or effector proteins into host cells.
Figures




References
-
- Abe, A., M. de Grado, R. A. Pfuetzner, C. Sanchez-Sanmartin, R. Devinney, J. L. Puente, N. C. Strynadka, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol. Microbiol. 33:1162-1175. - PubMed
-
- Combet, C., C. Blanchet, C. Geourjon, and G. Deleage. 2000. NPS@: network protein sequence analysis. Trends Biochem. Sci. 25:147-150. - PubMed
-
- Crawford, J. A., and J. B. Kaper. 2002. The N-terminus of enteropathogenic Escherichia coli (EPEC) Tir mediates transport across bacterial and eukaryotic cell membranes. Mol. Microbiol. 46:855-868. - PubMed
-
- Creasey, E. A., R. M. Delahay, A. A. Bishop, R. K. Shaw, B. Kenny, S. Knutton, and G. Frankel. 2003. CesT is a bivalent enteropathogenic Escherichia coli chaperone required for translocation of both Tir and Map. Mol. Microbiol. 47:209-221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

