Escherichia coli serogroup O107/O117 lipopolysaccharide binds and neutralizes Shiga toxin 2
- PMID: 15292153
- PMCID: PMC490921
- DOI: 10.1128/JB.186.16.5506-5512.2004
Escherichia coli serogroup O107/O117 lipopolysaccharide binds and neutralizes Shiga toxin 2
Abstract
The AB(5) toxin Shiga toxin 2 (Stx2) has been implicated as a major virulence factor of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains in the progression of intestinal disease to more severe systemic complications. Here, we demonstrate that supernatant from a normal E. coli isolate, FI-29, neutralizes the effect of Stx2, but not the related Stx1, on Vero cells. Biochemical characterization of the neutralizing activity identified the lipopolysaccharide (LPS) of FI-29, a serogroup O107/O117 strain, as the toxin-neutralizing component. LPSs from FI-29 as well as from type strains E. coli O107 and E. coli O117 were able bind Stx2 but not Stx1, indicating that the mechanism of toxin neutralization may involve inhibition of the interaction between Stx2 and the Gb(3) receptor on Vero cells.
Figures





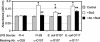

References
-
- Armstrong, G. D., P. C. Rowe, P. Goodyer, E. Orrbine, T. P. Klassen, G. Wells, A. MacKenzie, H. Lior, C. Blanchard, F. Auclair, et al. 1995. A phase I study of chemically synthesized verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to chromosorb for preventing hemolytic-uremic syndrome. J. Infect. Dis. 171:1042-1045. - PubMed
-
- Endo, Y., K. Tsurugi, T. Yutsudo, Y. Takeda, T. Ogasawara, and K. Igarashi. 1988. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171:45-50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

