Localization of RNAs to the mitochondrial cloud in Xenopus oocytes through entrapment and association with endoplasmic reticulum
- PMID: 15292452
- PMCID: PMC519158
- DOI: 10.1091/mbc.e04-03-0265
Localization of RNAs to the mitochondrial cloud in Xenopus oocytes through entrapment and association with endoplasmic reticulum
Abstract
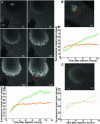
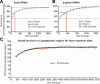
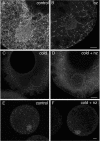
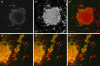
The germ cell lineage in Xenopus is specified by the inheritance of germ plasm, which originates within a distinct "mitochondrial cloud" (MC) in previtellogenic oocytes. Germ plasm contains localized RNAs implicated in germ cell development, including Xcat2 and Xdazl. To understand the mechanism of the early pathway through which RNAs localize to the MC, we applied live confocal imaging and photobleaching analysis to oocytes microinjected with fluorescent Xcat2 and Xdazl RNA constructs. These RNAs dispersed evenly throughout the cytoplasm through diffusion and then became progressively immobilized and formed aggregates in the MC. Entrapment in the MC was not prevented by microtubule disruption and did not require localization to germinal granules. Immobilized RNA constructs codistributed and showed coordinated movement with densely packed endoplasmic reticulum (ER) concentrated in the MC, as revealed with Dil16(3) labeling and immunofluorescence analysis. Vg1RBP/Vera protein, which has been implicated in linking late pathway RNAs to vegetal ER, was shown to bind specifically both wild-type Xcat2 3' untranslated region and localization-defective constructs. We found endogenous Vg1RBP/Vera and Vg1RBP/Vera-green fluorescent protein to be largely excluded from the MC but subsequently to codistribute with Xcat2 and ER at the vegetal cortex. We conclude that germ line RNAs localize into the MC through a diffusion/entrapment mechanism involving Vg1RBP/Vera-independent association with ER.
Figures



References
-
- Alarcon, V.B., and Elinson, R.P. (2001). RNA anchoring in the vegetal cortex of the Xenopus oocyte. J. Cell Sci. 114, 1731-1741. - PubMed
-
- Argon, Y., and Simen, B.B. (1999). GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol. 10, 495-505. - PubMed
-
- Bashirullah, A., Cooperstock, R.L., and Lipshitz, H.D. (1998). RNA localization in development. Annu. Rev. Biochem. 67, 335-394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

