Elevated endosomal cholesterol levels in Niemann-Pick cells inhibit rab4 and perturb membrane recycling
- PMID: 15292453
- PMCID: PMC519144
- DOI: 10.1091/mbc.e04-05-0432
Elevated endosomal cholesterol levels in Niemann-Pick cells inhibit rab4 and perturb membrane recycling
Abstract
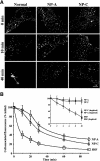
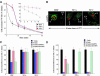
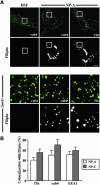
In normal human skin fibroblasts (HSFs), fluorescent glycosphingolipid analogues are endocytosed and sorted into two pools, one that is recycled to the plasma membrane and one that is transported to the Golgi complex. Here, we investigated glycosphingolipid recycling in Niemann-Pick type A and C lipid storage disease fibroblasts (NPFs). Cells were incubated with a fluorescent analogue of lactosylceramide (LacCer) at 16 degrees C to label early endosomes (EEs), shifted to 37 degrees C, and lipid recycling was quantified. Using dominant negative rabs, we showed that, in normal HSFs, LacCer recycling was rapid (t1/2 approximately 8 min) and mainly rab4-dependent. In NPFs, LacCer recycling was delayed (t1/2 approximately 30-40 min), and rab4-dependent recycling was absent, whereas rab11-dependent recycling predominated. Transferrin recycling via the rab4 pathway was similarly perturbed in NPFs. Compared with normal HSFs, EEs in NPFs showed high cholesterol levels and an altered organization of rab4. In vitro extraction of rab4 (but not rab11) with GDP dissociation inhibitor was severely attenuated in NPF endosomal fractions. This impairment was reversed with cholesterol depletion of isolated endosomes or with high-salt treatment of endosomes. These data suggest that abnormal membrane recycling in NPFs results from specific inhibition of rab4 function by excess cholesterol in EEs.
Figures




References
-
- Bligh, E.G., and Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911-917. - PubMed
-
- Cavalli, V., Vilbois, F., Corti, M., Marcote, M.J., Tamura, K., Karin, M., Arkinstall, S., and Gruenberg, J. (2001). The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol. Cell 7, 421-432. - PubMed
-
- Chen, C.S., Patterson, M.C., Wheatley, C.L., O'Brien, J.F., and Pagano, R.E. (1999). Broad screening test for sphingolipid-storage diseases. Lancet 354, 901-905. - PubMed
-
- Choudhury, A., Dominguez, M., Puri, V., Sharma, D.K., Narita, K., Wheatley, C.W., Marks, D.L., and Pagano, R.E. (2002). Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 109, 1541-1550. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

