De novo design of catalytic proteins
- PMID: 15292507
- PMCID: PMC511021
- DOI: 10.1073/pnas.0404387101
De novo design of catalytic proteins
Abstract
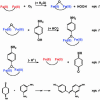
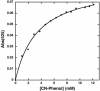
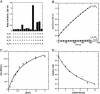
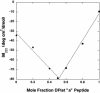
The de novo design of catalytic proteins provides a stringent test of our understanding of enzyme function, while simultaneously laying the groundwork for the design of novel catalysts. Here we describe the design of an O(2)-dependent phenol oxidase whose structure, sequence, and activity are designed from first principles. The protein catalyzes the two-electron oxidation of 4-aminophenol (k(cat)/K(M) = 1,500 M(-1).min(-1)) to the corresponding quinone monoimine by using a diiron cofactor. The catalytic efficiency is sensitive to changes of the size of a methyl group in the protein, illustrating the specificity of the design.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

