The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors
- PMID: 15294014
- PMCID: PMC1134038
- DOI: 10.1042/BJ20040984
The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors
Abstract
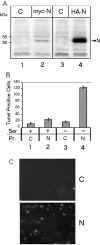
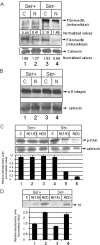
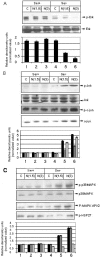
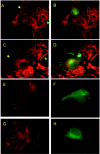
In March 2003, a novel coronavirus was isolated from patients exhibiting atypical pneumonia, and was subsequently proven to be the causative agent of the disease now referred to as SARS (severe acute respiratory syndrome). The complete genome of the SARS-CoV (SARS coronavirus) has since been sequenced. The SARS-CoV nucleocapsid (SARS-CoV N) protein shares little homology with other members of the coronavirus family. In the present paper, we show that SARS-CoV N is capable of inducing apoptosis of COS-1 monkey kidney cells in the absence of growth factors by down-regulating ERK (extracellular-signal-regulated kinase), up-regulating JNK (c-Jun N-terminal kinase) and p38 MAPK (mitogen-activated protein kinase) pathways, and affecting their downstream effectors. SARS-CoV N expression also down-regulated phospho-Akt and Bcl-2 levels, and activated caspases 3 and 7. However, apoptosis was independent of the p53 and Fas signalling pathways. Furthermore, activation of the p38 MAPK pathway was found to induce actin reorganization in cells devoid of growth factors. At the cytoskeletal level, SARS-CoV N down-regulated FAK (focal adhesion kinase) activity and also down-regulated fibronectin expression. This is the first report showing the ability of the N protein of SARS-CoV to induce apoptosis and actin reorganization in mammalian cells under stressed conditions.
Figures

Similar articles
-
Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase.J Cell Biol. 2000 May 1;149(3):741-54. doi: 10.1083/jcb.149.3.741. J Cell Biol. 2000. PMID: 10791986 Free PMC article.
-
Human intestinal epithelial crypt cell survival and death: Complex modulations of Bcl-2 homologs by Fak, PI3-K/Akt-1, MEK/Erk, and p38 signaling pathways.J Cell Physiol. 2004 Feb;198(2):209-22. doi: 10.1002/jcp.10399. J Cell Physiol. 2004. PMID: 14603523
-
JNK and PI3k/Akt signaling pathways are required for establishing persistent SARS-CoV infection in Vero E6 cells.Biochim Biophys Acta. 2005 Jun 30;1741(1-2):4-10. doi: 10.1016/j.bbadis.2005.04.004. Biochim Biophys Acta. 2005. PMID: 15916886 Free PMC article.
-
Signal transduction in SARS-CoV-infected cells.Ann N Y Acad Sci. 2007 Apr;1102(1):86-95. doi: 10.1196/annals.1408.006. Ann N Y Acad Sci. 2007. PMID: 17470913 Free PMC article. Review.
-
Serine/threonine protein kinases and apoptosis.Exp Cell Res. 2000 Apr 10;256(1):34-41. doi: 10.1006/excr.2000.4836. Exp Cell Res. 2000. PMID: 10739649 Review.
Cited by
-
Vitamin D binding protein in COVID-19.Clin Med (Lond). 2020 Sep;20(5):e136-e137. doi: 10.7861/clinmed.Let.20.5.2. Clin Med (Lond). 2020. PMID: 32934054 Free PMC article. No abstract available.
-
The molecular virology of coronaviruses.J Biol Chem. 2020 Sep 11;295(37):12910-12934. doi: 10.1074/jbc.REV120.013930. Epub 2020 Jul 13. J Biol Chem. 2020. PMID: 32661197 Free PMC article. Review.
-
The dimer interface of the SARS coronavirus nucleocapsid protein adapts a porcine respiratory and reproductive syndrome virus-like structure.FEBS Lett. 2005 Oct 24;579(25):5663-8. doi: 10.1016/j.febslet.2005.09.038. Epub 2005 Sep 30. FEBS Lett. 2005. PMID: 16214138 Free PMC article.
-
Severe acute respiratory syndrome coronavirus 3C-like protease-induced apoptosis.FEMS Immunol Med Microbiol. 2006 Apr;46(3):375-80. doi: 10.1111/j.1574-695X.2006.00045.x. FEMS Immunol Med Microbiol. 2006. PMID: 16553810 Free PMC article.
-
Severe acute respiratory syndrome coronavirus gene 7 products contribute to virus-induced apoptosis.J Virol. 2007 Oct;81(20):11054-68. doi: 10.1128/JVI.01266-07. Epub 2007 Aug 8. J Virol. 2007. PMID: 17686858 Free PMC article.
References
-
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H. R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R. A., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. - PubMed
-
- Ksiazek T. G., Erdman D., Goldsmith C. S., Zaki S. R., Peret T., Emery S., Tong S., Urbani C., Comer J. A., Lim W., et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. - PubMed
-
- Rota P. A., Oberste M. S., Monroe S. S., Nix W. A., Campagnoli R., Icenogle J. P., Penaranda S., Bankamp B., Maher K., Chen M. H., et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. - PubMed
-
- Marra M. A., Jones S. J., Astell C. R., Holt R. A., Brooks-Wilson A., Butterfield Y. S., Khattra J., Asano J. K., Barber S. A., Chan S. Y., et al. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

