Expression and immunogenicity of a recombinant diphtheria toxin fragment A in Streptococcus gordonii
- PMID: 15294787
- PMCID: PMC492408
- DOI: 10.1128/AEM.70.8.4569-4574.2004
Expression and immunogenicity of a recombinant diphtheria toxin fragment A in Streptococcus gordonii
Abstract
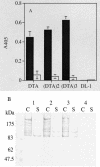
A nontoxic mutant diphtheria toxin fragment A (DTA) was genetically fused in single, double, or triple copy to the major surface protein antigen P1 (SpaP) and surface expressed in Streptococcus gordonii DL-1. The expression was verified by Western immunoblotting. Mouse antisera raised against the recombinant S. gordonii recognized the native diphtheria toxinm suggesting the recombinant DTA was immunogenic. When given intranasally to mice with cholera toxin subunit B as the adjuvant, the recombinant S. gordonii expressing double copies of DTA (SpaP-DTA(2)) induced a mucosal immunoglobulin A response and a weak systemic immunoglobulin G response. S. gordonii SpaP-DTA(2) was able to orally colonize BALB/c mice for a 15-week period and elicited a mucosal response, but a serum immunoglobulin G response was not apparent. The antisera failed to neutralize diphtheria toxin cytotoxicity in a Vero cell assay.
Figures

Similar articles
-
Expression of diphtheria toxin in Streptococcus mutans and induction of toxin-neutralizing antisera.Can J Microbiol. 2005 Oct;51(10):841-6. doi: 10.1139/w05-078. Can J Microbiol. 2005. PMID: 16333343
-
Expression of a pertussis toxin S1 fragment by inducible promoters in oral Streptococcus and the induction of immune responses during oral colonization in mice.Can J Microbiol. 2006 May;52(5):436-44. doi: 10.1139/w05-151. Can J Microbiol. 2006. PMID: 16699568
-
Oral colonization and immune responses to Streptococcus gordonii expressing a pertussis toxin S1 fragment in mice.FEMS Microbiol Lett. 2002 Mar 5;208(2):175-8. doi: 10.1111/j.1574-6968.2002.tb11078.x. FEMS Microbiol Lett. 2002. PMID: 11959433
-
Oral colonization and immune responses to Streptococcus gordonii: Potential use as a vector to induce antibodies against respiratory pathogens.Curr Opin Infect Dis. 2003 Jun;16(3):231-5. doi: 10.1097/00001432-200306000-00008. Curr Opin Infect Dis. 2003. PMID: 12821813 Review.
-
Lactic acid bacteria as live vaccines.Curr Issues Mol Biol. 2000 Jan;2(1):17-25. Curr Issues Mol Biol. 2000. PMID: 11464916 Review.
Cited by
-
Exploring Host-Commensal Interactions in the Respiratory Tract.Front Immunol. 2018 Jan 17;8:1971. doi: 10.3389/fimmu.2017.01971. eCollection 2017. Front Immunol. 2018. PMID: 29387057 Free PMC article. Review.
-
Expression and purification of truncated diphtheria toxin, DT386, in Escherichia coli: An attempt for production of a new vaccine against diphtheria.Res Pharm Sci. 2016 Oct;11(5):428-434. doi: 10.4103/1735-5362.192496. Res Pharm Sci. 2016. PMID: 27920826 Free PMC article.
-
Role of D-alanylation of Streptococcus gordonii lipoteichoic acid in innate and adaptive immunity.Infect Immun. 2007 Jun;75(6):3033-42. doi: 10.1128/IAI.01549-06. Epub 2007 Apr 9. Infect Immun. 2007. PMID: 17420241 Free PMC article.
-
Expression of a functional single-chain variable-fragment antibody against complement receptor 1 in Streptococcus gordonii.Clin Vaccine Immunol. 2008 Jun;15(6):925-31. doi: 10.1128/CVI.00500-07. Epub 2008 Apr 2. Clin Vaccine Immunol. 2008. PMID: 18385459 Free PMC article.
-
Construction and characterization of single-chain variable fragment antibodies directed against the Bordetella pertussis surface adhesins filamentous hemagglutinin and pertactin.Infect Immun. 2007 Nov;75(11):5476-82. doi: 10.1128/IAI.00494-07. Epub 2007 Aug 27. Infect Immun. 2007. PMID: 17724067 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

