Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes
- PMID: 15294788
- PMCID: PMC492325
- DOI: 10.1128/AEM.70.8.4575-4581.2004
Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes
Abstract
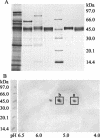
Agrocybe aegerita, a bark mulch- and wood-colonizing basidiomycete, was found to produce a peroxidase (AaP) that oxidizes aryl alcohols, such as veratryl and benzyl alcohols, into the corresponding aldehydes and then into benzoic acids. The enzyme also catalyzed the oxidation of typical peroxidase substrates, such as 2,6-dimethoxyphenol (DMP) or 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS). A. aegerita peroxidase production depended on the concentration of organic nitrogen in the medium, and highest enzyme levels were detected in the presence of soybean meal. Two fractions of the enzyme, AaP I and AaP II, which had identical molecular masses (46 kDa) and isoelectric points of 4.6 to 5.4 and 4.9 to 5.6, respectively (corresponding to six different isoforms), were identified after several steps of purification, including anion- and cation-exchange chromatography. The optimum pH for the oxidation of aryl alcohols was found to be around 7, and the enzyme required relatively high concentrations of H(2)O(2) (2 mM) for optimum activity. The apparent K(m) values for ABTS, DMP, benzyl alcohol, veratryl alcohol, and H(2)O(2) were 37, 298, 1,001, 2,367 and 1,313 microM, respectively. The N-terminal amino acid sequences of the main AaP II spots blotted after two-dimensional gel electrophoresis were almost identical and exhibited almost no homology to the sequences of other peroxidases from basidiomycetes, but they shared the first three amino acids, as well as two additional amino acids, with the heme chloroperoxidase (CPO) from the ascomycete Caldariomyces fumago. This finding is consistent with the fact that AaP halogenates monochlorodimedone, the specific substrate of CPO. The existence of haloperoxidases in basidiomycetous fungi may be of general significance for the natural formation of chlorinated organic compounds in forest soils.
Figures



Similar articles
-
The coprophilous mushroom Coprinus radians secretes a haloperoxidase that catalyzes aromatic peroxygenation.Appl Environ Microbiol. 2007 Sep;73(17):5477-85. doi: 10.1128/AEM.00026-07. Epub 2007 Jun 29. Appl Environ Microbiol. 2007. PMID: 17601809 Free PMC article.
-
Heme-thiolate haloperoxidases: versatile biocatalysts with biotechnological and environmental significance.Appl Microbiol Biotechnol. 2006 Jul;71(3):276-88. doi: 10.1007/s00253-006-0417-3. Appl Microbiol Biotechnol. 2006. PMID: 16628447 Review.
-
Peroxide oxidation of primary alcohols to aldehydes by chloroperoxidase catalysis.Biochem Biophys Res Commun. 1983 Aug 12;114(3):1104-8. doi: 10.1016/0006-291x(83)90676-9. Biochem Biophys Res Commun. 1983. PMID: 6615505
-
The haloperoxidase of the agaric fungus Agrocybe aegerita hydroxylates toluene and naphthalene.FEBS Lett. 2005 Nov 7;579(27):6247-50. doi: 10.1016/j.febslet.2005.10.014. Epub 2005 Oct 19. FEBS Lett. 2005. PMID: 16253244
-
Structural and functional comparisons between vanadium haloperoxidase and acid phosphatase enzymes.J Mol Recognit. 2002 Sep-Oct;15(5):291-6. doi: 10.1002/jmr.590. J Mol Recognit. 2002. PMID: 12447906 Review.
Cited by
-
Driving force for oxygen-atom transfer by heme-thiolate enzymes.Angew Chem Int Ed Engl. 2013 Aug 26;52(35):9238-41. doi: 10.1002/anie.201302137. Epub 2013 Jul 3. Angew Chem Int Ed Engl. 2013. PMID: 23825007 Free PMC article. No abstract available.
-
Transcriptome and proteome exploration to provide a resource for the study of Agrocybe aegerita.PLoS One. 2013;8(2):e56686. doi: 10.1371/journal.pone.0056686. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418592 Free PMC article.
-
Water-Soluble Anthraquinone Photocatalysts Enable Methanol-Driven Enzymatic Halogenation and Hydroxylation Reactions.ACS Catal. 2020 Aug 7;10(15):8277-8284. doi: 10.1021/acscatal.0c01958. Epub 2020 Jun 30. ACS Catal. 2020. PMID: 32802571 Free PMC article.
-
Genome sequencing and molecular networking analysis of the wild fungus Anthostomella pinea reveal its ability to produce a diverse range of secondary metabolites.Fungal Biol Biotechnol. 2024 Jan 3;11(1):1. doi: 10.1186/s40694-023-00170-1. Fungal Biol Biotechnol. 2024. PMID: 38172933 Free PMC article.
-
Synthesis of cyclophosphamide metabolites by a peroxygenase from Marasmius rotula for toxicological studies on human cancer cells.AMB Express. 2020 Jul 18;10(1):128. doi: 10.1186/s13568-020-01064-w. AMB Express. 2020. PMID: 32683510 Free PMC article.
References
-
- Baciocchi, E., M. Fabbrini, O. Lanzalunga, L. Manduchi, and G. Pochetti. 2001. Prochiral selectivity in H2O2-promoted oxidation of arylalcanols catalysed by chloroperoxidase. Eur. J. Biochem. 268:665-672. - PubMed
-
- Bar-Nun, N., S. Shcolnick, and A. M. Mayer. 2002. Presence of a vanadium-dependent haloperoxidase in Botrytis cinerea. FEMS Microbiol. Lett. 217:121-124. - PubMed
-
- Conesa, A., F. van de Velde, F. van Rantwijk, R. A. Sheldon, C. A. M. J. J. van den Hondel, and P. J. Punt. 2001. Expression of the Caldariomyces fumago chloroperoxidase in Aspergillus niger and characterization of the recombinant enzyme. J. Biol. Chem. 276:17635-17640. - PubMed
-
- Dawson, J. H., and M. Sono. 1987. Cytochrome P-450 and chloroperoxidase:thiolate-ligated heme enzymes. Spectroscopic determination of their active site structures and mechanistic implications of thiolate ligation. Chem. Rev. 87:1255-1276.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

