Expression of a heterologous manganese superoxide dismutase gene in intestinal lactobacilli provides protection against hydrogen peroxide toxicity
- PMID: 15294805
- PMCID: PMC492360
- DOI: 10.1128/AEM.70.8.4702-4710.2004
Expression of a heterologous manganese superoxide dismutase gene in intestinal lactobacilli provides protection against hydrogen peroxide toxicity
Abstract
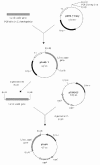
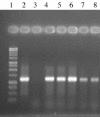
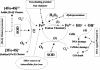
In living organisms, exposure to oxygen provokes oxidative stress. A widespread mechanism for protection against oxidative stress is provided by the antioxidant enzymes: superoxide dismutases (SODs) and hydroperoxidases. Generally, these enzymes are not present in Lactobacillus spp. In this study, we examined the potential advantages of providing a heterologous SOD to some of the intestinal lactobacilli. Thus, the gene encoding the manganese-containing SOD (sodA) was cloned from Streptococcus thermophilus AO54 and expressed in four intestinal lactobacilli. A 1.2-kb PCR product containing the sodA gene was cloned into the shuttle vector pTRK563, to yield pSodA, which was functionally expressed and complemented an Escherichia coli strain deficient in Mn and FeSODs. The plasmid, pSodA, was subsequently introduced and expressed in Lactobacillus gasseri NCK334, Lactobacillus johnsonii NCK89, Lactobacillus acidophilus NCK56, and Lactobacillus reuteri NCK932. Molecular and biochemical analyses confirmed the presence of the gene (sodA) and the expression of an active gene product (MnSOD) in these strains of lactobacilli. The specific activities of MnSOD were 6.7, 3.8, 5.8, and 60.7 U/mg of protein for L. gasseri, L. johnsonii, L. acidophilus, and L. reuteri, respectively. The expression of S. thermophilus MnSOD in L. gasseri and L. acidophilus provided protection against hydrogen peroxide stress. The data show that MnSOD protects cells against hydrogen peroxide by removing O(2)(.-) and preventing the redox cycling of iron. To our best knowledge, this is the first report of a sodA from S. thermophilus being expressed in other lactic acid bacteria.
Figures



References
-
- Andrus, J. M., S. W. Bowen, T. R. Klaenhammer, and H. M. Hassan. 2003. Molecular characterization and functional analysis of the manganese-containing superoxide dismutase gene (sodA) from Streptococcus thermophilus AO54. Arch. Biochem. Biophys. 420:103-113. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1989. Short protocols in molecular biology. Green Publishing Associates and Wiley Interscience, New York, N.Y.
-
- Axelsson, L. 1998. Lactic acid bacteria: classification and physiology, p. 1-12. In S. Salminen and A. von Wright (ed.), Lactic acid bacteria: microbiology and functional aspects. Marcel Dekker, Inc., New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

