Synaptic strengthening mediated by bone morphogenetic protein-dependent retrograde signaling in the Drosophila CNS
- PMID: 15295025
- PMCID: PMC6729602
- DOI: 10.1523/JNEUROSCI.1978-04.2004
Synaptic strengthening mediated by bone morphogenetic protein-dependent retrograde signaling in the Drosophila CNS
Abstract
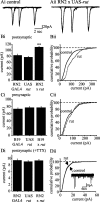
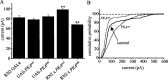
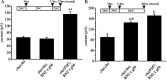
Retrograde signaling is an essential component of synaptic development and physiology. Previous studies show that bone morphogenetic protein (BMP)-dependent retrograde signaling is required for the proper development of the neuromuscular junction (NMJ) in Drosophila. These studies, moreover, raised the significant possibility that the development of central motor circuitry might similarly be reliant on such signaling. To test this hypothesis, retrograde signaling between postsynaptic motoneurons and their presynaptic interneurons is examined. Postsynaptic expression of an adenylate cyclase encoded by rutabaga (rut), is sufficient to strengthen synaptic transmission at these identified central synapses. Results are presented to show that the underlying mechanism is dependent on BMP retrograde signaling. Thus, presynaptic expression of an activated TGF-beta receptor, thickvien (tkv), or postsynaptic expression of a TGF-beta ligand, glass-bottom boat (gbb), is sufficient to phenocopy strengthening of synaptic transmission. In the absence of gbb, endogenous synaptic transmission is significantly weakened and, moreover, postsynaptic overexpression of rut is unable to potentiate synaptic function. Potentiation of presynaptic neurotransmitter release, mediated by increased postsynaptic expression of gbb, is dependent on normal cholinergic activity, indicative that either the secretion of this retrograde signal, or its transduction, is activity dependent. Thus, in addition to the development of the NMJ and expression of myoactive FMRFamide-like peptides in specific central neurons, the results of the present study indicate that this retrograde signaling cascade also integrates the development and function of central motor circuitry that controls movement in Drosophila larvae.
Figures



References
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS (2002) Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33: 545-558. - PubMed
-
- Allan DW, St Pierre SE, Miguel-Aliaga I, Thor S (2003) Specification of neuropeptide cell identity by the integration of retrograde BMP signalling and a combinatorial transcription factor code. Cell 113: 73-86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
