Autocatalytic self-propagation of misfolded prion protein
- PMID: 15297610
- PMCID: PMC514458
- DOI: 10.1073/pnas.0404650101
Autocatalytic self-propagation of misfolded prion protein
Abstract
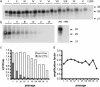
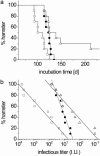
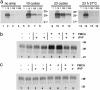
Prions are thought to replicate in an autocatalytic process that converts cellular prion protein (PrP(C)) to the disease-associated misfolded PrP isoform (PrP(Sc)). Our study scrutinizes this hypothesis by in vitro protein misfolding cyclic amplification (PMCA). In serial transmission PMCA experiments, PrP(Sc) was inoculated into healthy hamster brain homogenate containing PrP(C). Misfolded PrP was amplified by rounds of sonication and incubation and reinoculated into fresh brain homogenate every 10 PMCA rounds. The amplification depended on PrP(C) substrate and could be inhibited by recombinant hamster PrP. In serial dilution experiments, newly formed misfolded and proteinase K-resistant PrP (PrPres) catalyzed the structural conversion of PrP(C) as efficiently as PrP(Sc) from brain of scrapie (263K)-infected hamsters, yielding an approximately 300-fold total amplification of PrPres after 100 rounds, which confirms an autocatalytic PrP-misfolding cascade as postulated by the prion hypothesis. PrPres formation was not paralleled by replication of biological infectivity, which appears to require factors additional to PrP-misfolding autocatalysis.
Figures

References
-
- Prusiner, S. B. (1982) Science 216, 136-144. - PubMed
-
- Kocisko, D. A., Priola, S. A., Raymond, G. J., Chesebro, B., Lansbury, P. T., Jr., & Caughey, B. (1994) Nature 370, 471-474. - PubMed
-
- Saborio, G. P., Permanne, B. & Soto, C. (2001) Nature 411, 810-813. - PubMed
-
- Jarrett, J. T. & Lansbury, P. T., Jr. (1993) Cell 73, 1055-1058. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

