Structural basis for the dual coding potential of 8-oxoguanosine by a high-fidelity DNA polymerase
- PMID: 15297882
- PMCID: PMC516626
- DOI: 10.1038/sj.emboj.7600354
Structural basis for the dual coding potential of 8-oxoguanosine by a high-fidelity DNA polymerase
Abstract
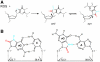
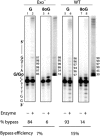
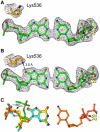
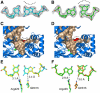
Accurate DNA replication involves polymerases with high nucleotide selectivity and proofreading activity. We show here why both fidelity mechanisms fail when normally accurate T7 DNA polymerase bypasses the common oxidative lesion 8-oxo-7, 8-dihydro-2'-deoxyguanosine (8oG). The crystal structure of the polymerase with 8oG templating dC insertion shows that the O8 oxygen is tolerated by strong kinking of the DNA template. A model of a corresponding structure with dATP predicts steric and electrostatic clashes that would reduce but not eliminate insertion of dA. The structure of a postinsertional complex shows 8oG(syn).dA (anti) in a Hoogsteen-like base pair at the 3' terminus, and polymerase interactions with the minor groove surface of the mismatch that mimic those with undamaged, matched base pairs. This explains why translesion synthesis is permitted without proofreading of an 8oG.dA mismatch, thus providing insight into the high mutagenic potential of 8oG.
Figures


References
-
- Beckman KB, Ames BN (1997) Oxidative decay of DNA. J Biol Chem 272: 19633–19636 - PubMed
-
- Boudsocq F, Kokoska RJ, Plosky BS, Vaisman A, Ling H, Kunkel TA, Yang W, Woodgate R (2004) Investigating the role of the little finger domain of Y-family DNA polymerases in low-fidelity synthesis and translesion replication. J Biol Chem, (in press) - PubMed
-
- Brautigam CA, Steitz TA (1998) Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes. Curr Opin Struct Biol 8: 54–63 - PubMed
-
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D 54: 905–921 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

