Logistics of community smallpox control through contact tracing and ring vaccination: a stochastic network model
- PMID: 15298713
- PMCID: PMC520756
- DOI: 10.1186/1471-2458-4-34
Logistics of community smallpox control through contact tracing and ring vaccination: a stochastic network model
Abstract
Background: Previous smallpox ring vaccination models based on contact tracing over a network suggest that ring vaccination would be effective, but have not explicitly included response logistics and limited numbers of vaccinators.
Methods: We developed a continuous-time stochastic simulation of smallpox transmission, including network structure, post-exposure vaccination, vaccination of contacts of contacts, limited response capacity, heterogeneity in symptoms and infectiousness, vaccination prior to the discontinuation of routine vaccination, more rapid diagnosis due to public awareness, surveillance of asymptomatic contacts, and isolation of cases.
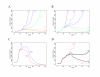
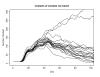
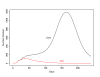
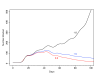
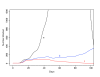
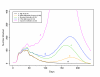
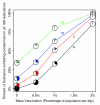
Results: We found that even in cases of very rapidly spreading smallpox, ring vaccination (when coupled with surveillance) is sufficient in most cases to eliminate smallpox quickly, assuming that 95% of household contacts are traced, 80% of workplace or social contacts are traced, and no casual contacts are traced, and that in most cases the ability to trace 1-5 individuals per day per index case is sufficient. If smallpox is assumed to be transmitted very quickly to contacts, it may at times escape containment by ring vaccination, but could be controlled in these circumstances by mass vaccination.
Conclusions: Small introductions of smallpox are likely to be easily contained by ring vaccination, provided contact tracing is feasible. Uncertainties in the nature of bioterrorist smallpox (infectiousness, vaccine efficacy) support continued planning for ring vaccination as well as mass vaccination. If initiated, ring vaccination should be conducted without delays in vaccination, should include contacts of contacts (whenever there is sufficient capacity) and should be accompanied by increased public awareness and surveillance.
Figures


References
-
- Alibek K. Biohazard. New York: Random House; 1999.
-
- Foege WH, Millar JD, Lane JM. Selective epidemiologic control in smallpox eradication. American Journal of Epidemiology. 1971;94:311–315. - PubMed

