A molecular dynamics study of Ca(2+)-calmodulin: evidence of interdomain coupling and structural collapse on the nanosecond timescale
- PMID: 15298887
- PMCID: PMC1304488
- DOI: 10.1529/biophysj.103.033266
A molecular dynamics study of Ca(2+)-calmodulin: evidence of interdomain coupling and structural collapse on the nanosecond timescale
Abstract
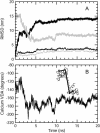
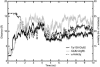
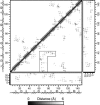
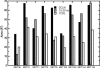
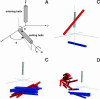
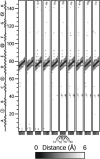
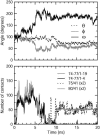
A 20-ns molecular dynamics simulation of Ca(2+)-calmodulin (CaM) in explicit solvent is described. Within 5 ns, the extended crystal structure adopts a compact shape similar in dimension to complexes of CaM and target peptides but with a substantially different orientation between the N- and C-terminal domains. Significant interactions are observed between the terminal domains in this compact state, which are mediated through the same regions of CaM that bind to target peptides derived from protein kinases and most other target proteins. The process of compaction is driven by the loss of helical structure in two separate regions between residues 75-79 and 82-86, the latter being driven by unfavorable electrostatic interactions between acidic residues. In the first 5 ns of the simulation, a substantial number of contacts are observed between the first helix of the N-terminal domain and residues 74-77 of the central linker. These contacts are correlated with the closing of the second EF-hand, indicating a mechanism by which they can lower calcium affinity in the N-terminal domain.
Figures



References
-
- Aliste, M., J. L. MacCallum, and D. P. Tieleman. 2003. Molecular dynamics simulations of pentapeptides at interfaces: salt bridge and cation-π interactions. Biochemistry. 42:8976–8987. - PubMed
-
- Babu, Y. S., C. E. Bugg, and W. J. Cook. 1988. Structure of calmodulin refined at 2.2 Å resolution. J. Mol. Biol. 204:191–204. - PubMed
-
- Barbato, G., M. Ikura, L. E. Kay, R. W. Pastor, and A. Bax. 1992. Backbone dynamics of calmodulin studied by 15N relaxation using inverse detected two-dimensional NMR spectroscopy: the central helix is flexible. Biochemistry. 31:5269–5278. - PubMed
-
- Barton, N. P., C. S. Verma, and L. S. A. Caves. 2002. Inherent flexibility of calmodulin domains: a normal-mode analysis study. J. Phys. Chem. B. 106:11036–11040.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

