A kinetic study of the growth of fatty acid vesicles
- PMID: 15298905
- PMCID: PMC1304506
- DOI: 10.1529/biophysj.104.039875
A kinetic study of the growth of fatty acid vesicles
Abstract
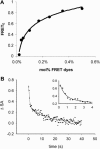
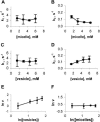
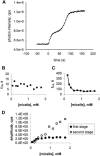
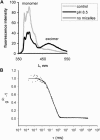
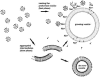
Membrane vesicles composed of fatty acids can be made to grow and divide under laboratory conditions, and thus provide a model system relevant to the emergence of cellular life. Fatty acid vesicles grow spontaneously when alkaline micelles are added to buffered vesicles. To investigate the mechanism of this process, we used stopped-flow kinetics to analyze the dilution of non-exchanging FRET probes incorporated into preformed vesicles during growth. Oleate vesicle growth occurs in two phases (fast and slow), indicating two pathways for the incorporation of fatty acid into preformed vesicles. We propose that the fast phase, which is stoichiometrically limited by the preformed vesicles, results from the formation of a "shell" of fatty acid around a vesicle, followed by rapid transfer of this fatty acid into the preformed vesicle. The slower phase may result from incorporation of fatty acid which had been trapped in an intermediate state. We provide independent evidence for the rapid transformation of micelles into an aggregated intermediate form after transfer from high to low pH. Our results show that the most efficient incorporation of added oleate into oleic acid/oleate vesicles occurs under conditions that avoid a large transient increase in the micelle/vesicle ratio.
Figures

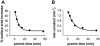
References
-
- Allahyarov, E., I. D‘Amico, and H. Lowen. 1998. Attraction between like-charged macroions by Coulomb depletion. Phys. Rev. Lett. 81:1334–1337.
-
- Allen, W. V., and C. Ponnamperuma. 1967. A possible prebiotic synthesis of monocarboxylic acids. Curr. Mod. Biol. 1:24–28. - PubMed
-
- Almgren, M., F. Grieser, and J. K. Thomas. 1979. Dynamic and static aspects of solubilization of neutral arenes in ionic micellar solutions. J. Am. Chem. Soc. 101:279–291.
-
- An, S. W., J. R. Lu, and R. K. Thomas. 1996. Apparent anomalies in surface excesses determined from neutron reflection and the Gibbs equation in anionic surfactants with particular reference to perfluorooctanoates at the air/water interface. Langmuir. 12:2446–2453.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

