The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers
- PMID: 15298907
- PMCID: PMC1304443
- DOI: 10.1529/biophysj.103.034280
The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers
Abstract
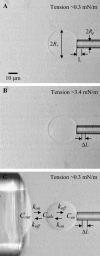
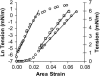
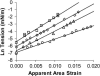
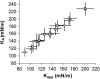
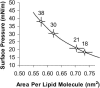
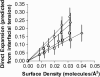
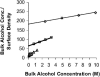
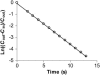
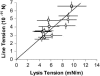
We used micropipette aspiration to directly measure the area compressibility modulus, bending modulus, lysis tension, lysis strain, and area expansion of fluid phase 1-stearoyl, 2-oleoyl phosphatidylcholine (SOPC) lipid bilayers exposed to aqueous solutions of short-chain alcohols at alcohol concentrations ranging from 0.1 to 9.8 M. The order of effectiveness in decreasing mechanical properties and increasing area per molecule was butanol>propanol>ethanol>methanol, although the lysis strain was invariant to alcohol chain-length. Quantitatively, the trend in area compressibility modulus follows Traube's rule of interfacial tension reduction, i.e., for each additional alcohol CH(2) group, the concentration required to reach the same area compressibility modulus was reduced roughly by a factor of 3. We convert our area compressibility data into interfacial tension values to: confirm that Traube's rule is followed for bilayers; show that alcohols decrease the interfacial tension of bilayer-water interfaces less effectively than oil-water interfaces; determine the partition coefficients and standard Gibbs adsorption energy per CH(2) group for adsorption of alcohol into the lipid headgroup region; and predict the increase in area per headgroup as well as the critical radius and line tension of a membrane pore for each concentration and chain-length of alcohol. The area expansion predictions were confirmed by direct measurements of the area expansion of vesicles exposed to flowing alcohol solutions. These measurements were fitted to a membrane kinetic model to find membrane permeability coefficients of short-chain alcohols. Taken together, the evidence presented here supports a view that alcohol partitioning into the bilayer headgroup region, with enhanced partitioning as the chain-length of the alcohol increases, results in chain-length-dependent interfacial tension reduction with concomitant chain-length-dependent reduction in mechanical moduli and membrane thickness.
Figures





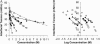
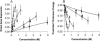


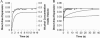
Similar articles
-
Effect of chain length and unsaturation on elasticity of lipid bilayers.Biophys J. 2000 Jul;79(1):328-39. doi: 10.1016/S0006-3495(00)76295-3. Biophys J. 2000. PMID: 10866959 Free PMC article.
-
Permeability and the hidden area of lipid bilayers.Eur Biophys J. 2004 Dec;33(8):706-14. doi: 10.1007/s00249-004-0415-2. Epub 2004 May 26. Eur Biophys J. 2004. PMID: 15164236
-
Measurement of membrane elasticity by micro-pipette aspiration.Eur Phys J E Soft Matter. 2004 Jun;14(2):149-67. doi: 10.1140/epje/i2003-10146-y. Eur Phys J E Soft Matter. 2004. PMID: 15254835
-
Micropipet aspiration for measuring elastic properties of lipid bilayers.Methods Mol Biol. 2007;400:421-37. doi: 10.1007/978-1-59745-519-0_28. Methods Mol Biol. 2007. PMID: 17951750 Review.
-
Exploration of lipid bilayer mechanical properties using molecular dynamics simulation.Arch Biochem Biophys. 2024 Nov;761:110151. doi: 10.1016/j.abb.2024.110151. Epub 2024 Sep 10. Arch Biochem Biophys. 2024. PMID: 39265694 Review.
Cited by
-
Thermodynamics of membrane elasticity--a molecular level approach to one- and two-component fluid amphiphilic membranes, part II: applications.Eur Phys J E Soft Matter. 2005 Feb;16(2):125-39. doi: 10.1140/epje/e2005-00014-1. Epub 2005 Feb 22. Eur Phys J E Soft Matter. 2005. PMID: 15729504
-
Physiological, Genetic, and Transcriptomic Analysis of Alcohol-Induced Delay of Escherichia coli Death.Appl Environ Microbiol. 2019 Jan 9;85(2):e02113-18. doi: 10.1128/AEM.02113-18. Print 2019 Jan 15. Appl Environ Microbiol. 2019. PMID: 30389772 Free PMC article.
-
Methanol Accelerates DMPC Flip-Flop and Transfer: A SANS Study on Lipid Dynamics.Biophys J. 2019 Mar 5;116(5):755-759. doi: 10.1016/j.bpj.2019.01.021. Epub 2019 Jan 29. Biophys J. 2019. PMID: 30777306 Free PMC article.
-
Complex Energy Landscapes of Self-Assembled Vesicles.J Am Chem Soc. 2023 Jul 19;145(28):15496-15506. doi: 10.1021/jacs.3c04285. Epub 2023 Jul 10. J Am Chem Soc. 2023. PMID: 37427769 Free PMC article.
-
Alcohols reduce lateral membrane pressures: predictions from molecular theory.Biophys J. 2006 Dec 1;91(11):4081-90. doi: 10.1529/biophysj.106.091918. Epub 2006 Sep 15. Biophys J. 2006. PMID: 16980354 Free PMC article.
References
-
- Adamson, A. W., and A. P. Gast. 1997. Physical Chemistry of Surfaces. Wiley, New York.
-
- Almeida, P. F. F., W. L. C. Vaz, and T. E. Thompson. 1992. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine cholesterol lipid bilayers—a free-volume analysis. Biochemistry. 31:6739–6747. - PubMed
-
- Angelova, M. I., R. Mutafchieva, R. Dimova, and B. Tenchov. 1999. Shape transformations of giant unilamellar vesicles induced by ethanol and temperature variations. Colloids Surfaces. 149:201–205.
-
- Angelova, M. I., S. Soleau, P. Meleard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Prog. Colloid Polym. Sci. 89:127–131.
-
- Apostoluk, W., and J. Szymanowski. 1998. Estimation of alcohol adsorption parameters at hydrocarbon/water interfaces in systems containing various hydrocarbons. Colloids Surfaces. 132:137–143.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

