hnRNP H binding at the 5' splice site correlates with the pathological effect of two intronic mutations in the NF-1 and TSHbeta genes
- PMID: 15299088
- PMCID: PMC514374
- DOI: 10.1093/nar/gkh752
hnRNP H binding at the 5' splice site correlates with the pathological effect of two intronic mutations in the NF-1 and TSHbeta genes
Abstract
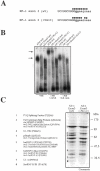
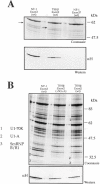
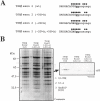
We have recently reported a disease-causing substitution (+5G > C) at the donor site of NF-1 exon 3 that produces its skipping. We have now studied in detail the splicing mechanism involved in analyzing RNA-protein complexes at several 5' splice sites. Characteristic protein patterns were observed by pulldown and band-shift/super-shift analysis. Here, we show that hnRNP H binds specifically to the wild-type GGGgu donor sequence of the NF-1 exon 3. Depletion analyses shows that this protein restricts the accessibility of U1 small nuclear ribonucleoprotein (U1snRNA) to the donor site. In this context, the +5G > C mutation abolishes both U1snRNP base pairing and the 5' splice site (5'ss) function. However, exon recognition in the mutant can be rescued by disrupting the binding of hnRNP H, demonstrating that this protein enhances the effects of the +5G > C substitution. Significantly, a similar situation was found for a second disease-causing +5G > A substitution in the 5'ss of TSHbeta exon 2, which harbors a GGgu donor sequence. Thus, the reason why similar nucleotide substitutions can be either neutral or very disruptive of splicing function can be explained by the presence of specific binding signatures depending on local contexts.
Figures





References
-
- Burge C.B., Tuschl,T. and Sharp,P.A. (1999) Splicing of Precursors to mRNAs by the Spliceosome. Cold Spring Laboratory Harbor Press, Cold Spring Harbor, NY.
-
- Mount S.M., Pettersson,I., Hinterberger,M., Karmas,A. and Steitz,J.A. (1983) The U1 small nuclear RNA–protein complex selectively binds a 5′ splice site in vitro. Cell, 33, 509–518. - PubMed
-
- Stark H., Dube,P., Luhrmann,R. and Kastner,B. (2001) Arrangement of RNA and proteins in the spliceosomal U1 small nuclear ribonucleoprotein particle. Nature, 409, 539–542. - PubMed
-
- Zhang M.Q. (1998) Statistical features of human exons and their flanking regions. Hum. Mol. Genet., 7, 919–932. - PubMed
-
- Michaud S. and Reed,R. (1991) An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes Dev., 5, 2534–2546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

