Transient release of oxygenated volatile organic compounds during light-dark transitions in Grey poplar leaves
- PMID: 15299129
- PMCID: PMC520768
- DOI: 10.1104/pp.104.043240
Transient release of oxygenated volatile organic compounds during light-dark transitions in Grey poplar leaves
Abstract
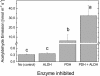
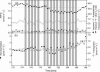
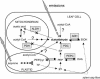
In this study, we investigated the prompt release of acetaldehyde and other oxygenated volatile organic compounds (VOCs) from leaves of Grey poplar [Populus x canescens (Aiton) Smith] following light-dark transitions. Mass scans utilizing the extremely fast and sensitive proton transfer reaction-mass spectrometry technique revealed the following temporal pattern after light-dark transitions: hexenal was emitted first, followed by acetaldehyde and other C(6)-VOCs. Under anoxic conditions, acetaldehyde was the only compound released after switching off the light. This clearly indicated that hexenal and other C(6)-VOCs were released from the lipoxygenase reaction taking place during light-dark transitions under aerobic conditions. Experiments with enzyme inhibitors that artificially increased cytosolic pyruvate demonstrated that the acetaldehyde burst after light-dark transition could not be explained by the recently suggested pyruvate overflow mechanism. The simulation of light fleck situations in the canopy by exposing leaves to alternating light-dark and dark-light transitions or fast changes from high to low photosynthetic photon flux density showed that this process is of minor importance for acetaldehyde emission into the Earth's atmosphere.
Figures





Similar articles
-
Green leaf volatiles and oxygenated metabolite emission bursts from mesquite branches following light-dark transitions.Photosynth Res. 2012 Sep;113(1-3):321-33. doi: 10.1007/s11120-012-9746-5. Epub 2012 Jun 19. Photosynth Res. 2012. PMID: 22711426
-
VOC emissions of Grey poplar leaves as affected by salt stress and different N sources.Plant Biol (Stuttg). 2008 Jan;10(1):86-96. doi: 10.1111/j.1438-8677.2007.00015.x. Plant Biol (Stuttg). 2008. PMID: 18211549
-
On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature.Plant Cell Environ. 2006 Sep;29(9):1820-8. doi: 10.1111/j.1365-3040.2006.01561.x. Plant Cell Environ. 2006. PMID: 16913871
-
Carbon isotope analysis of acetaldehyde emitted from leaves following mechanical stress and anoxia.Plant Biol (Stuttg). 2009 Jul;11(4):591-7. doi: 10.1111/j.1438-8677.2008.00155.x. Plant Biol (Stuttg). 2009. PMID: 19538397
-
Volatile signaling in plant-plant interactions: "talking trees" in the genomics era.Science. 2006 Feb 10;311(5762):812-5. doi: 10.1126/science.1118446. Science. 2006. PMID: 16469918 Review.
Cited by
-
Green leaf volatiles and oxygenated metabolite emission bursts from mesquite branches following light-dark transitions.Photosynth Res. 2012 Sep;113(1-3):321-33. doi: 10.1007/s11120-012-9746-5. Epub 2012 Jun 19. Photosynth Res. 2012. PMID: 22711426
-
Olfactory Response of the Spotted Asparagus Beetle, Crioceris duodecimpunctata (L.) to Host Plant Volatiles.J Chem Ecol. 2022 Jan;48(1):41-50. doi: 10.1007/s10886-021-01323-5. Epub 2021 Nov 5. J Chem Ecol. 2022. PMID: 34738203
-
Spectacular Oscillations in Plant Isoprene Emission under Transient Conditions Explain the Enigmatic CO2 Response.Plant Physiol. 2016 Dec;172(4):2275-2285. doi: 10.1104/pp.16.01002. Epub 2016 Oct 21. Plant Physiol. 2016. PMID: 27770061 Free PMC article.
-
Biogenic volatile organic compound and respiratory CO2 emissions after 13C-labeling: online tracing of C translocation dynamics in poplar plants.PLoS One. 2011 Feb 28;6(2):e17393. doi: 10.1371/journal.pone.0017393. PLoS One. 2011. PMID: 21387007 Free PMC article.
-
Diurnal and seasonal variation of isoprene biosynthesis-related genes in grey poplar leaves.Plant Physiol. 2005 Sep;139(1):474-84. doi: 10.1104/pp.105.066373. Epub 2005 Aug 26. Plant Physiol. 2005. PMID: 16126852 Free PMC article.
References
-
- Bate NJ, Rothstein SJ (1998) C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J 16: 561–569 - PubMed
-
- Bode K, Helas G, Kesselmeier J (1997) Biogenic contribution to atmospheric organic acids. In G Helas, J Slanina, R Steinbrecher, eds, Biogenic Volatile Organic Compounds in the Atmosphere. SPB Academic Publishing, Amsterdam, pp 157–170
-
- Brasseur GP, Chatfield RB (1991) The fate of biogenic trace gases in the atmosphere. In TD Sharkey, B Holland, HA Mooney, eds, Trace Gas Emissions by Plants. Academic Press, New York, pp 1–28
-
- Charron CS, Cantliffe DJ, Wheeler RM, Manukian A, Heath RR (1996) A system and methodology for measuring volatile organic compounds produced by hydroponic lettuce in a controlled environment. J Am Soc Hortic Sci 121: 483–487 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

