Differential antifungal and calcium channel-blocking activity among structurally related plant defensins
- PMID: 15299136
- PMCID: PMC520777
- DOI: 10.1104/pp.104.040873
Differential antifungal and calcium channel-blocking activity among structurally related plant defensins
Abstract
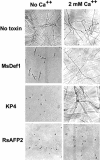
Plant defensins are a family of small Cys-rich antifungal proteins that play important roles in plant defense against invading fungi. Structures of several plant defensins share a Cys-stabilized alpha/beta-motif. Structural determinants in plant defensins that govern their antifungal activity and the mechanisms by which they inhibit fungal growth remain unclear. Alfalfa (Medicago sativa) seed defensin, MsDef1, strongly inhibits the growth of Fusarium graminearum in vitro, and its antifungal activity is markedly reduced in the presence of Ca(2+). By contrast, MtDef2 from Medicago truncatula, which shares 65% amino acid sequence identity with MsDef1, lacks antifungal activity against F. graminearum. Characterization of the in vitro antifungal activity of the chimeras containing portions of the MsDef1 and MtDef2 proteins shows that the major determinants of antifungal activity reside in the carboxy-terminal region (amino acids 31-45) of MsDef1. We further define the active site by demonstrating that the Arg at position 38 of MsDef1 is critical for its antifungal activity. Furthermore, we have found for the first time, to our knowledge, that MsDef1 blocks the mammalian L-type Ca(2+) channel in a manner akin to a virally encoded and structurally unrelated antifungal toxin KP4 from Ustilago maydis, whereas structurally similar MtDef2 and the radish (Raphanus sativus) seed defensin Rs-AFP2 fail to block the L-type Ca(2+) channel. From these results, we speculate that the two unrelated antifungal proteins, KP4 and MsDef1, have evolutionarily converged upon the same molecular target, whereas the two structurally related antifungal plant defensins, MtDef2 and Rs-AFP2, have diverged to attack different targets in fungi.
Figures




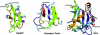
References
-
- Almeida MS, Cabral KM, Kurtenbach E, Almeida FC, Valente AP (2002) Solution structure of Pisum sativum defensin 1 by high resolution NMR: plant defensins, identical backbone with different mechanisms of action. J Mol Biol 315: 749–757 - PubMed
-
- Bloch C, Richardson M (1991) A new family of small (5 kDa) protein inhibitors of insect alpha-amylases from seeds of Sorghum (Sorghum bicolor (L) Moench) have sequence homologies with wheat gamma-purothionins. FEBS Lett 279: 101–105 - PubMed
-
- Bontems F, Roumestand C, Boyot P, Gilquin B, Doljansky Y, Menez A, Toma F (1991) Three-dimensional structure of natural charybdotoxin in aqueous solution by 1H-NMR. Charybdotoxin possesses a structural motif found in other scorpion toxins. Eur J Biochem 196: 19–28 - PubMed
-
- Borkovich KA, Alex LA, Yarden O, Freitag M, Turner GE, Read ND, Seiler S, Bell-Pedersen D, Paietta J, Plesofsky N, et al (2004) Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev 68: 1–108 (table of contents) - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

