Disorganization of cortical microtubules stimulates tangential expansion and reduces the uniformity of cellulose microfibril alignment among cells in the root of Arabidopsis
- PMID: 15299138
- PMCID: PMC520797
- DOI: 10.1104/pp.104.040493
Disorganization of cortical microtubules stimulates tangential expansion and reduces the uniformity of cellulose microfibril alignment among cells in the root of Arabidopsis
Abstract
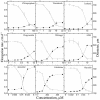
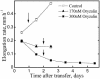
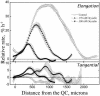
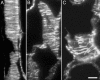
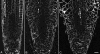
To test the role of cortical microtubules in aligning cellulose microfibrils and controlling anisotropic expansion, we exposed Arabidopsis thaliana roots to moderate levels of the microtubule inhibitor, oryzalin. After 2 d of treatment, roots grow at approximately steady state. At that time, the spatial profiles of relative expansion rate in length and diameter were quantified, and roots were cryofixed, freeze-substituted, embedded in plastic, and sectioned. The angular distribution of microtubules as a function of distance from the tip was quantified from antitubulin immunofluorescence images. In alternate sections, the overall amount of alignment among microfibrils and their mean orientation as a function of position was quantified with polarized-light microscopy. The spatial profiles of relative expansion show that the drug affects relative elongation and tangential expansion rates independently. The microtubule distributions averaged to transverse in the growth zone for all treatments, but on oryzalin the distributions became broad, indicating poorly organized arrays. At a subcellular scale, cellulose microfibrils in oryzalin-treated roots were as well aligned as in controls; however, the mean alignment direction, while consistently transverse in the controls, was increasingly variable with oryzalin concentration, meaning that microfibril orientation in one location tended to differ from that of a neighboring location. This conclusion was confirmed by direct observations of microfibrils with field-emission scanning electron microscopy. Taken together, these results suggest that cortical microtubules ensure microfibrils are aligned consistently across the organ, thereby endowing the organ with a uniform mechanical structure.
Figures





References
-
- Baskin TI (2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215: 150–171 - PubMed
-
- Baskin TI, Beemster GTS (1998) On the “postmitotic isodiametric growth zone” in roots. In HE Flores, J Lynch, D Eissenstat, eds, Radical Biology, Advances and Perspectives on the Function of Plant Roots. American Society of Plant Physiologists, Rockville MD, pp 23–33
-
- Baskin TI, Miller DD, Vos JW, Wilson JE, Hepler PK (1996) Cryofixing single cells and multicellular specimens enhances structure and immunocytochemistry for light microscopy. J Microsc 182: 149–161 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

