Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation
- PMID: 15299142
- PMCID: PMC520785
- DOI: 10.1104/pp.103.037929
Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation
Abstract
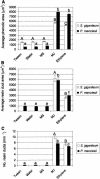
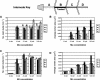
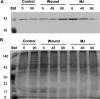
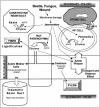
Conifer stem pest resistance includes constitutive defenses that discourage invasion and inducible defenses, including phenolic and terpenoid resin synthesis. Recently, methyl jasmonate (MJ) was shown to induce conifer resin and phenolic defenses; however, it is not known if MJ is the direct effector or if there is a downstream signal. Exogenous applications of MJ, methyl salicylate, and ethylene were used to assess inducible defense signaling mechanisms in conifer stems. MJ and ethylene but not methyl salicylate caused enhanced phenolic synthesis in polyphenolic parenchyma cells, early sclereid lignification, and reprogramming of the cambial zone to form traumatic resin ducts in Pseudotsuga menziesii and Sequoiadendron giganteum. Similar responses in internodes above and below treated internodes indicate transport of a signal giving a systemic response. Studies focusing on P. menziesii showed MJ induced ethylene production earlier and 77-fold higher than wounding. Ethylene production was also induced in internodes above the MJ-treated internode. Pretreatment of P. menziesii stems with the ethylene response inhibitor 1-methylcyclopropene inhibited MJ and wound responses. Wounding increased 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase protein, but MJ treatment produced a higher and more rapid ACC oxidase increase. ACC oxidase was most abundant in ray parenchyma cells, followed by cambial zone cells and resin duct epithelia. The data show these MJ-induced defense responses are mediated by ethylene. The cambial zone xylem mother cells are reprogrammed to differentiate into resin-secreting epithelial cells by an MJ-induced ethylene burst, whereas polyphenolic parenchyma cells are activated to increase polyphenol production. The results also indicate a central role of ray parenchyma in ethylene-induced defense.
Figures





Similar articles
-
Methyl jasmonate induces changes mimicking anatomical defenses in diverse members of the Pinaceae.Tree Physiol. 2003 Apr;23(6):361-71. doi: 10.1093/treephys/23.6.361. Tree Physiol. 2003. PMID: 12642238
-
Induction of anatomically based defense responses in stems of diverse conifers by methyl jasmonate: a phylogenetic perspective.Tree Physiol. 2004 Mar;24(3):251-64. doi: 10.1093/treephys/24.3.251. Tree Physiol. 2004. PMID: 14704135
-
Ethylene in induced conifer defense: cDNA cloning, protein expression, and cellular and subcellular localization of 1-aminocyclopropane-1-carboxylate oxidase in resin duct and phenolic parenchyma cells.Planta. 2006 Sep;224(4):865-77. doi: 10.1007/s00425-006-0274-4. Epub 2006 May 17. Planta. 2006. PMID: 16705404
-
Aminocyclopropane carboxylic acid synthase is a regulated step in ethylene-dependent induced conifer defense. Full-length cDNA cloning of a multigene family, differential constitutive, and wound- and insect-induced expression, and cellular and subcellular localization in spruce and Douglas fir.Plant Physiol. 2007 Jan;143(1):410-24. doi: 10.1104/pp.106.089425. Epub 2006 Nov 22. Plant Physiol. 2007. PMID: 17122070 Free PMC article.
-
Terpenoid biosynthesis and specialized vascular cells of conifer defense.J Integr Plant Biol. 2010 Jan;52(1):86-97. doi: 10.1111/j.1744-7909.2010.00910.x. J Integr Plant Biol. 2010. PMID: 20074143 Review.
Cited by
-
Using the CODIT model to explain secondary metabolites of xylem in defence systems of temperate trees against decay fungi.Ann Bot. 2020 Apr 25;125(5):701-720. doi: 10.1093/aob/mcz138. Ann Bot. 2020. PMID: 31420666 Free PMC article. Review.
-
Roles of ethylene, jasmonic acid, and salicylic acid and their interactions in frankincense resin production in Boswellia sacra Flueck. trees.Sci Rep. 2020 Oct 7;10(1):16760. doi: 10.1038/s41598-020-73993-2. Sci Rep. 2020. PMID: 33028915 Free PMC article.
-
Common plantain. A collection of expressed sequence tags from vascular tissue and a simple and efficient transformation method.Plant Physiol. 2006 Dec;142(4):1427-41. doi: 10.1104/pp.106.089169. Epub 2006 Oct 13. Plant Physiol. 2006. PMID: 17041024 Free PMC article.
-
Exogenous application of methyl jasmonate elicits defenses in Norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus.Oecologia. 2006 Jun;148(3):426-36. doi: 10.1007/s00442-006-0394-3. Epub 2006 Mar 3. Oecologia. 2006. PMID: 16514534
-
Roles of JnRAP2.6-like from the transition zone of black walnut in hormone signaling.PLoS One. 2013 Nov 12;8(11):e75857. doi: 10.1371/journal.pone.0075857. eCollection 2013. PLoS One. 2013. PMID: 24265672 Free PMC article.
References
-
- Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego, pp 26–55
-
- Alfaro RI (1995) An induced defense reaction in white spruce to attack by the white pine weevil (Pissodes strobi). Can J For Res 25: 1725–1730
-
- Andersson-Gunneras S, Hellgren JM, Bjorklund S, Regan S, Moritz T, Sundberg B (2003) Asymmetric expression of a poplar ACC oxidase controls ethylene production during gravitational induction of tension wood. Plant J 34: 339–349 - PubMed
-
- Bannan MW (1936) Vertical resin ducts in the secondary wood of the Abietineae. New Phytol 35: 11–46
-
- Barker JE (1979) Growth and wood properties of Pinus radiata in relation to applied ethylene. N Z J For Sci 9: 15–19
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

