CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection
- PMID: 15300249
- PMCID: PMC2776074
- DOI: 10.1038/ni1105
CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection
Abstract
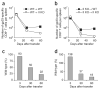
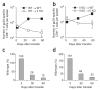
Immunization in the absence of CD4(+) T cell help results in defective CD8(+) T cell memory, deficient recall responses and diminished protective immunity. Here we investigated at what stage during the immune response to pathogen CD4(+) T cells are essential in the promotion of functional CD8(+) T cell memory. Memory CD8(+) T cell numbers decreased gradually in the absence of CD4(+) T cells despite the presence of similar numbers of memory cell precursors at the peak of the effector phase. Adoptive transfer of effector or memory CD8(+) T cells into wild-type or CD4(+) T cell-deficient mice demonstrated that the presence of CD4(+) T cells was important only after, not during, the early CD8(+) T cell programming phase. In the absence of CD4(+) T cells, memory CD8(+) T cells became functionally impaired and decreased in quantity over time. We conclude that in the context of an acute infection, CD4(+) T cells are required only during the maintenance phase of long-lived memory CD8(+) T cells.
Figures
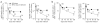


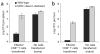
Comment in
-
Environmental conservation: bystander CD4 T cells keep CD8 memories fresh.Nat Immunol. 2004 Sep;5(9):873-4. doi: 10.1038/ni0904-873. Nat Immunol. 2004. PMID: 15334079 No abstract available.
Similar articles
-
Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection.J Immunol. 2008 Mar 1;180(5):2855-62. doi: 10.4049/jimmunol.180.5.2855. J Immunol. 2008. PMID: 18292507
-
CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses.Proc Natl Acad Sci U S A. 2010 Jan 5;107(1):193-8. doi: 10.1073/pnas.0909945107. Epub 2009 Dec 4. Proc Natl Acad Sci U S A. 2010. PMID: 19966302 Free PMC article.
-
Requirement for CD4 T cell help in generating functional CD8 T cell memory.Science. 2003 Apr 11;300(5617):337-9. doi: 10.1126/science.1082305. Science. 2003. PMID: 12690201
-
Induction and maintenance of CD8+ T cells specific for persistent viruses.Adv Exp Med Biol. 2007;590:121-37. doi: 10.1007/978-0-387-34814-8_9. Adv Exp Med Biol. 2007. PMID: 17191382 Review. No abstract available.
-
Memory CD4 T cells: generation, reactivation and re-assignment.Immunology. 2010 May;130(1):10-5. doi: 10.1111/j.1365-2567.2010.03260.x. Epub 2010 Mar 16. Immunology. 2010. PMID: 20331469 Free PMC article. Review.
Cited by
-
CD4(+) T-cell dependence of primary CD8(+) T-cell response against vaccinia virus depends upon route of infection and viral dose.Cell Mol Immunol. 2016 Jan;13(1):82-93. doi: 10.1038/cmi.2014.128. Epub 2014 Dec 29. Cell Mol Immunol. 2016. PMID: 25544501 Free PMC article.
-
Persistent Antigen and Prolonged AKT-mTORC1 Activation Underlie Memory CD8 T Cell Impairment in the Absence of CD4 T Cells.J Immunol. 2015 Aug 15;195(4):1591-8. doi: 10.4049/jimmunol.1500451. Epub 2015 Jul 10. J Immunol. 2015. PMID: 26163589 Free PMC article.
-
Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis.Nat Med. 2005 Apr;11(4 Suppl):S33-44. doi: 10.1038/nm1221. Nat Med. 2005. PMID: 15812488 Free PMC article. Review.
-
CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells.Br J Cancer. 2007 Jun 18;96(12):1839-48. doi: 10.1038/sj.bjc.6603792. Epub 2007 May 15. Br J Cancer. 2007. PMID: 17505510 Free PMC article.
-
Organ- and disease-stage-specific regulation of Toxoplasma gondii-specific CD8-T-cell responses by CD4 T cells.Infect Immun. 2006 Oct;74(10):5790-801. doi: 10.1128/IAI.00098-06. Infect Immun. 2006. PMID: 16988257 Free PMC article.
References
-
- Mercado R, et al. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. - PubMed
-
- van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. - PubMed
-
- van Stipdonk MJ, et al. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. - PubMed
-
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8+ T cells after infection. Nat Immunol. 2002;3:619–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

