Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: electrophysiologic, biochemical, and morphologic features
- PMID: 15302661
- PMCID: PMC2865831
- DOI: 10.1001/archopht.122.8.1190
Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: electrophysiologic, biochemical, and morphologic features
Abstract
Objective: To assess the electrophysiologic, histologic, and biochemical features of an animal model of Smith-Lemli-Opitz syndrome (SLOS).
Methods: Sprague-Dawley rats were treated with AY9944, a selective inhibitor of 3beta-hydroxysterol-Delta(7)-reductase (the affected enzyme in SLOS). Dark- and light-adapted electroretinograms were obtained from treated and control animals. From each animal, 1 retina was analyzed by microscopy, and the contralateral retina plus serum samples were analyzed for sterol composition. The main outcome measures were rod and cone electroretinographic amplitudes and implicit times, outer nuclear layer (ONL) thickness, rod outer segment length, pyknotic ONL nucleus counts, and the 7-dehydrocholesterol/cholesterol mole ratio in the retina and serum.
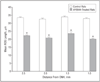
Results: By 10 weeks' postnatal age, rod and cone electroretinographic wave amplitudes in AY9944-treated animals were significantly reduced and implicit times were significantly increased relative to controls. Maximal rod photoresponse and gain values were reduced approximately 2-fold in treated animals relative to controls. The ONL thickness and average rod outer segment length were reduced by approximately 18% and 33%, respectively, and ONL pyknotic nucleus counts were approximately 4.5-fold greater in treated animals relative to controls. The retinal pigment epithelium of treated animals contained massive amounts of membranous/lipid inclusions not routinely observed in controls. The 7-dehydrocholesterol/cholesterol mole ratios in treated retinas and serum samples were approximately 5:1 and 9:1, respectively, whereas the ratios in control tissues were essentially zero.
Conclusions: This rodent model exhibits the key biochemical hallmarks associated with SLOS and displays electrophysiologic deficits comparable to or greater than those observed in the human disease. Clinical Relevance These results predict retinal degeneration in patients with SLOS, particularly those with the more severe (type II) form of the disease, and may be more broadly relevant to other inborn errors of cholesterol biosynthesis. This animal model may also be of use in evaluating therapeutic treatments for SLOS and in understanding the slow phototransduction kinetics observed in patients with SLOS.
Figures







References
-
- Smith JD, Lemli L, Opitz JM. A newly recognized syndrome of multiple congenital anomalies. J Pediatr. 1964;64:210–217. - PubMed
-
- Kelley RI. Inborn errors of cholesterol biosynthesis. Adv Pediatr. 2000;47:1–53. - PubMed
-
- Porter FD. Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr Opin Pediatr. 2003;15:607–613. - PubMed
-
- Herman GE. Disorders of cholesterol biosynthesis: prototypic metabolic malformation syndromes. Hum Mol Genet. 2003;12 suppl 1:R75–R88. - PubMed
-
- Battaile KP, Battaile BC, Merkens LS, Maslen CL, Steiner RD. Carrier frequency of the common mutation IVS8-1G>C in DHCR7 and estimate of the expected incidence of Smith-Lemli-Opitz syndrome. Mol Genet Metab. 2001;72:67–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions

