Multiple triclosan targets in Trypanosoma brucei
- PMID: 15302818
- PMCID: PMC500884
- DOI: 10.1128/EC.3.4.855-861.2004
Multiple triclosan targets in Trypanosoma brucei
Abstract
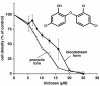
Trypanosoma brucei genes encoding putative fatty acid synthesis enzymes are homologous to those encoding type II enzymes found in bacteria and organelles such as chloroplasts and mitochondria. It was therefore not surprising that triclosan, an inhibitor of type II enoyl-acyl carrier protein (enoyl-ACP) reductase, killed both procyclic forms and bloodstream forms of T. brucei in culture with 50% effective concentrations (EC(50)s) of 10 and 13 microM, respectively. Triclosan also inhibited cell-free fatty acid synthesis, though much higher concentrations were required (EC(50)s of 100 to 200 microM). Unexpectedly, 100 microM triclosan did not affect the elongation of [(3)H]laurate (C(12:0)) to myristate (C(14:0)) in cultured bloodstream form parasites, suggesting that triclosan killing of trypanosomes may not be through specific inhibition of enoyl-ACP reductase but through some other mechanism. Interestingly, 100 microM triclosan did reduce the level of incorporation of [(3)H]myristate into glycosyl phosphatidylinositol species (GPIs). Furthermore, we found that triclosan inhibited fatty acid remodeling in a cell-free assay in the same concentration range required for killing T. brucei in culture. In addition, we found that a similar concentration of triclosan also inhibited the myristate exchange pathway, which resides in a distinct subcellular compartment. However, GPI myristoylation and myristate exchange are specific to the bloodstream form parasite, yet triclosan kills both the bloodstream and procyclic forms. Therefore, triclosan killing may be due to a nonspecific perturbation of subcellular membrane structure leading to dysfunction in sensitive membrane-resident biochemical pathways.
Figures

References
-
- Bhargava, H. N., and P. A. Leonard. 1996. Triclosan: applications and safety. Am. J. Infect. Control 24:209-218. - PubMed
-
- Brun, R., and M. Shonenberger. 1979. Cultivation and in vitro cloning of procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 36:289-292. - PubMed
-
- Buxbaum, L. U., J. Raper, F. R. Opperdoes, and P. T. Englund. 1994. Myristate exchange: a second glycosyl phosphatidylinositol myristoylation reaction in African trypanosomes. J. Biol. Chem. 269:30212-30220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

