The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK
- PMID: 15302853
- PMCID: PMC2172212
- DOI: 10.1083/jcb.200404012
The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK
Abstract
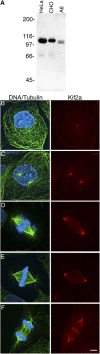
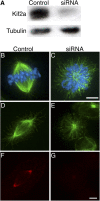
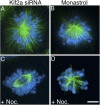
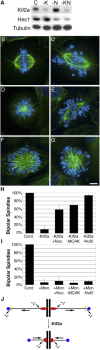
Although the microtubule-depolymerizing KinI motor Kif2a is abundantly expressed in neuronal cells, we now show it localizes to centrosomes and spindle poles during mitosis in cultured cells. RNAi-induced knockdown of Kif2a expression inhibited cell cycle progression because cells assembled monopolar spindles. Bipolar spindle assembly was restored in cells lacking Kif2a by treatments that altered microtubule assembly (nocodazole), eliminated kinetochore-microtubule attachment (loss of Nuf2), or stabilized microtubule plus ends at kinetochores (loss of MCAK). Thus, two KinI motors, MCAK and Kif2a, play distinct roles in mitosis, and MCAK activity at kinetochores must be balanced by Kif2a activity at poles for spindle bipolarity. These treatments failed to restore bipolarity to cells lacking the activity of the kinesin Eg5. Thus, two independent pathways contribute to spindle bipolarity, with the Eg5-dependent pathway using motor force to drive spindle bipolarity and the Kif2a-dependent pathway relying on microtubule polymer dynamics to generate force for spindle bipolarity.
Figures

References
-
- Compton, D.A. 2000. Spindle assembly in animal cells. Annu. Rev. Biochem. 69:95–114. - PubMed
-
- Debernardi, S., E. Fontanella, L. De Gregorio, M.A. Pierotti, and D. Delia. 1997. Identification of a novel human kinesin-related gene (HK2) by the cDNA differential display technique. Genomics. 42:67–73. - PubMed
-
- DeLuca, J.G., B.J. Howell, J.C. Canman, J.M. Hickey, G. Fang, and E.D. Salmon. 2003. Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr. Biol. 13:2103–2109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

