Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice
- PMID: 15302904
- PMCID: PMC2211927
- DOI: 10.1084/jem.20040342
Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice
Abstract
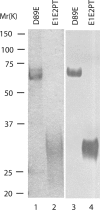
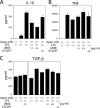
Apoptotic cells are rapidly phagocytosed by professional phagocytes, such as macrophages and dendritic cells. This process prevents the release of potentially noxious or immunogenic intracellular materials from dying cells, and is thought to play a critical role for the maintenance of normal functions in surrounding tissues. Milk fat globule-EGF-factor 8 (MFG-E8), secreted by activated macrophages and immature dendritic cells, links apoptotic cells and phagocytes, and promotes phagocytosis of apoptotic cells. Here, we report that an MFG-E8 mutant, designated as D89E, carrying a point mutation in an RGD motif, inhibited not only the phagocytosis of apoptotic cells by a wide variety of phagocytes, but also inhibited the enhanced production of IL-10 by thioglycollate-elicited peritoneal macrophages phagocytosing apoptotic cells. When intravenously injected into mice, the D89E protein induced the production of autoantibodies including antiphospholipids antibodies and antinuclear antibodies. The production of autoantibodies was enhanced by the coinjection of syngeneic apoptotic thymocytes. After the induction of autoantibody production by D89E, the treated mice showed a long-term elevation of the titer for autoantibodies, and developed IgG deposition in the glomeruli. These results indicated that the impairment of apoptotic cell phagocytosis led to autoantibody production.
Figures






References
-
- Jacobson, M.D., M. Weil, and M.C. Raff. 1997. Programmed cell death in animal development. Cell. 88:347–354. - PubMed
-
- Vaux, D.L., and S.J. Korsmeyer. 1999. Cell death in development. Cell. 96:245–254. - PubMed
-
- Stroh, C., and K. Schulze-Osthoff. 1998. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 5:997–1000. - PubMed
-
- Nagata, S. 1997. Apoptosis by death factor. Cell. 88:355–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

