Evolution of p53 in hypoxia-stressed Spalax mimics human tumor mutation
- PMID: 15302922
- PMCID: PMC514462
- DOI: 10.1073/pnas.0404998101
Evolution of p53 in hypoxia-stressed Spalax mimics human tumor mutation
Abstract
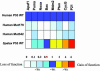
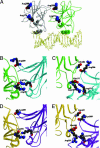
The tumor suppressor gene p53 controls cellular response to a variety of stress conditions, including DNA damage and hypoxia, leading to growth arrest and/or apoptosis. Inactivation of p53, found in 40-50% of human cancers, confers selective advantage under hypoxic microenvironment during tumor progression. The mole rat, Spalax, spends its entire life cycle underground at decidedly lower oxygen tensions than any other mammal studied. Because a wide range of respiratory adaptations to hypoxic stress evolved in Spalax, we speculated that it might also have developed hypoxia adaptation mechanisms analogous to the genetic/epigenetic alterations acquired during tumor progression. Comparing Spalax with human and mouse p53 revealed an arginine (R) to lysine (K) substitution in Spalax (Arg-174 in human) in the DNA-binding domain, identical to known tumor associated mutations. Multiple p53 sequence alignments with 41 additional species confirmed that Arg-174 is highly conserved. Reporter assays uncovered that Spalax p53 protein is unable to induce apoptosis-regulating target genes, resulting in no expression of apaf1 and partial expression of puma, pten, and noxa. However, cell cycle arrest and p53 stabilization/homeostasis genes were overactivated by Spalax p53. Lys-174 was found critical for apaf1 expression inactivation. A DNA-free p53 structure model predicts that Arg-174 is important for dimerization, whereas Spalax Lys-174 prevents such interactions. Similar neighboring mutations found in human tumors favor growth arrest rather than apoptosis. We hypothesize that, in an analogy with human tumor progression, Spalax underwent remarkable adaptive p53 evolution during 40 million years of underground hypoxic life.
Figures


References
-
- Klein, G. (2004) Cell Death Differ. 11, 13-17. - PubMed
-
- Vousden, K. H. & Lu, X. (2002) Nat. Rev. Cancer 2, 594-604. - PubMed
-
- Levine, A. J. (1997) Cell 88, 323-331. - PubMed
-
- Graeber, T. G., Osmanian, C., Jacks, T., Housman, D. E., Koch, C. J., Lowe, S. W. & Giaccia, A. J. (1996) Nature 379, 88-91. - PubMed
-
- Kinzler, K. W. & Vogelstein, B. (1996) Nature 379, 19-20. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

