A vascular cell-restricted RhoGAP, p73RhoGAP, is a key regulator of angiogenesis
- PMID: 15302923
- PMCID: PMC514459
- DOI: 10.1073/pnas.0404631101
A vascular cell-restricted RhoGAP, p73RhoGAP, is a key regulator of angiogenesis
Abstract
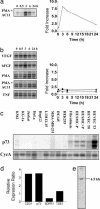
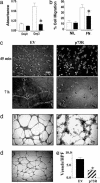
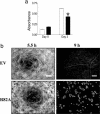
Angiogenesis is a major therapeutic target. Ideal drug targets are genes expressed only in endothelial cells (ECs) or only during the angiogenic process. Here, we describe a gene, p73RhoGAP (p73), that has both of these properties. By using a PCR-based subtraction-hybridization approach to clone cDNAs from ECs undergoing capillary-tube formation, we identified a RhoGAP member, p73. p73 displays GTPase activity to Rho but not to Rac or Cdc42. Knockdown of p73 protein, achieved by adenovirus delivery of p73 antisense and by small interfering RNA into ECs, demonstrated the importance of this protein in EC function. Under such conditions, EC migration, proliferation, and capillary-tube formation were inhibited. Furthermore, angiogenesis in vivo was also inhibited by antisense p73. A mutant R82A alteration achieved a similar phenotype in vitro to the antisense, demonstrating the importance of the GTPase-activating protein activity to p73 function. Expression profiling of p73 shows that it is vascular cell-selective, being highly expressed in ECs and smooth-muscle cells but not in other cell types. Finally, we show that the mRNA of p73 is up-regulated in an angiogenic milieu with little or no regulation seen under nonangiogenic conditions. p73, a vascular cell-specific GTPase-activating protein, is an important modulator of angiogenesis and displays many of features that make it worthy of being a drug target.
Figures


References
-
- Augustin, H. G. (1998) Trends Pharmacol. Sci. 19, 216-222. - PubMed
-
- Hanahan, D. (1997) Science 277, 48-50. - PubMed
-
- Gamble, J., Meyer, G., Noack, L., Furze, J., Matthias, L., Kovach, N., Harlant, J. & Vadas, M. (1999) Endothelium 7, 23-34. - PubMed
-
- Bayless, K. J. & Davis, G. E. (2002) J. Cell Sci. 115, 1123-1136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

