The essence of total synthesis
- PMID: 15302925
- PMCID: PMC514411
- DOI: 10.1073/pnas.0403799101
The essence of total synthesis
Abstract
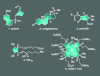
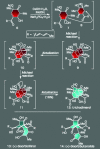
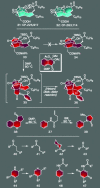
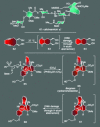
For the past century, the total synthesis of natural products has served as the flagship of chemical synthesis and the principal driving force for discovering new chemical reactivity, evaluating physical organic theories, testing the power of existing synthetic methods, and enabling biology and medicine. This perspective article seeks to examine this time-honored and highly demanding art, distilling its essence in an effort to ascertain its power and future potential.
Figures


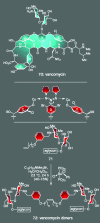


References
-
- Nicolaou, K. C. & Sorensen, E. J. (1996) Classics in Total Synthesis: Targets, Strategies, Methods (VCH, Weinheim, Germany), p. 799.
-
- Nicolaou, K. C. & Snyder, S. A. (2003) Classics in Total Synthesis II: More Targets, Strategies, Methods (Wiley–VCH, Weinheim, Germany), p. 639.
-
- Nicolaou, K. C., Vourloumis, D., Winssinger, N. & Baran, P. S. (2000) Angew. Chem. Int. Ed. 39, 44-122. - PubMed
-
- Garfield, S. (2000) Mauve (Faber & Faber, London), p. 224.
-
- Djerassi, C. (1990) Steroids Made It Possible (American Chemical Society, Washington. D.C.), p. 205.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

