DNA targeting of rhinal cortex D2 receptor protein reversibly blocks learning of cues that predict reward
- PMID: 15302926
- PMCID: PMC514406
- DOI: 10.1073/pnas.0403639101
DNA targeting of rhinal cortex D2 receptor protein reversibly blocks learning of cues that predict reward
Abstract
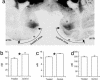
When schedules of several operant trials must be successfully completed to obtain a reward, monkeys quickly learn to adjust their behavioral performance by using visual cues that signal how many trials have been completed and how many remain in the current schedule. Bilateral rhinal (perirhinal and entorhinal) cortex ablations irreversibly prevent this learning. Here, we apply a recombinant DNA technique to investigate the role of dopamine D2 receptor in rhinal cortex for this type of learning. Rhinal cortex was injected with a DNA construct that significantly decreased D2 receptor ligand binding and temporarily produced the same profound learning deficit seen after ablation. However, unlike after ablation, the D2 receptor-targeted, DNA-treated monkeys recovered cue-related learning after 11-19 weeks. Injecting a DNA construct that decreased N-methyl-d-aspartate but not D2 receptor ligand binding did not interfere with learning associations between the cues and the schedules. A second D2 receptor-targeted DNA treatment administered after either recovery from a first D2 receptor-targeted DNA treatment (one monkey), after N-methyl-d-aspartate receptor-targeted DNA treatment (two monkeys), or after a vector control treatment (one monkey) also induced a learning deficit of similar duration. These results suggest that the D2 receptor in primate rhinal cortex is essential for learning to relate the visual cues to the schedules. The specificity of the receptor manipulation reported here suggests that this approach could be generalized in this or other brain pathways to relate molecular mechanisms to cognitive functions.
Figures

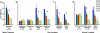
References
-
- Bowman, E. M., Aigner, T. G. & Richmond, B. J. (1996) J. Neurophysiol. 75, 1061–1073. - PubMed
-
- Liu, Z., Murray, E. A. & Richmond, B. J. (2000) Nat. Neurosci. 3, 1307–1315. - PubMed
-
- Liu, Z. & Richmond, B. J. (2000) J. Neurophysiol. 83, 1677–1692. - PubMed
-
- Shidara, M. & Richmond, B. J. (2002) Science 296, 1623–1624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

