Silencing the ap65 gene reduces adherence to vaginal epithelial cells by Trichomonas vaginalis
- PMID: 15306014
- PMCID: PMC2562645
- DOI: 10.1111/j.1365-2958.2004.04192.x
Silencing the ap65 gene reduces adherence to vaginal epithelial cells by Trichomonas vaginalis
Abstract
Host parasitism by Trichomonas vaginalis is complex and in part mediated by adherence to vaginal epithelial cells (VECs). Four trichomonad surface proteins bind VECs as adhesins, and AP65 is a major adhesin with sequence identity to an enzyme of the hydrogenosome organelle that is involved in energy generation. In order to perform genetic analysis and assess the role of AP65 in T. vaginalis adherence, we silenced expression of ap65 using antisense RNA. The gene for ap65 was inserted into the vector pBS-neo in sense and antisense orientations to generate plasmids pBS-neoS (S) and pBS-neoAS (AS), respectively. Trichomonads were then transfected with S and AS plasmids for selection of stable transfectants using Geneticin, and the presence of plasmid in transfectants was confirmed by polymerase chain reaction of the neo gene. Reverse transcription polymerase chain reaction and Northern blot analysis showed decreased amounts of ap65 transcript in AS transfected parasites. Growth kinetics of the antisense-transfected and wild type organisms were similar, suggesting that silencing AP65 did not affect overall energy generation for growth. Immunoblot analysis using monoclonal antibody (mAb) to AP65 of AS transfectants showed decreased amounts of AP65 when compared to wild type or S transfectants. Not unexpectedly, this corresponded to decreased amounts of AP65 bound to VECs in a functional ligand assay. Reduction in parasite surface expression of AP65 was related to lower levels of adherence to VECs by AS-transfectants compared to control organisms. Antisense silencing of ap65 was not alleviated by growth of trichomonads in high iron, which up-regulates transcription of ap65. Our work reaffirms the role for AP65 as an adhesin, and in addition, we demonstrate antisense RNA gene silencing in T. vaginalis to study the contribution of specific genes in pathogenesis.
Figures





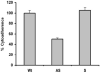
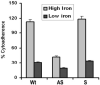
References
-
- Alderete JF, Engbring J, Lauriano CM, O’Brien JL. Only two of the Trichomonas vaginalis triplet AP51 adhesins are regulated by iron. Microb Pathog. 1998;23:1–16. - PubMed
-
- Alderete JF, Millsap KW, Lehker MW, Benchimol M. Enzymes on microbial pathogens and Trichomonas vaginalis: molecular mimicry and functional diversity. Cell Microbiol. 2001;3:1–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

