Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis
- PMID: 15306653
- PMCID: PMC6729180
- DOI: 10.1523/JNEUROSCI.1933-04.2004
Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis
Abstract
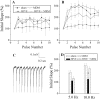
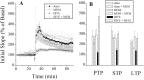
Memantine, a low-to-moderate-affinity NMDA receptor antagonist, can be used to treat cognitive impairment associated with Alzheimer's disease. However, its potential neuroprotective effects for human immunodeficiency virus type 1-associated (HIV-1-associated) dementia are less well appreciated. To this end we studied hippocampal synaptic function in a severe combined immunodeficient (SCID) mouse model of HIV-1 encephalitis (HIVE). Human monocyte-derived macrophages (MDMs) infected with HIV-1(ADA) were injected stereotactically into the caudate and putamen of SCID mice, generating HIVE. These brain subregions are among those most affected in humans. Impaired synaptic transmission and long-term potentiation (LTP) were detected in the CA1 region of hippocampal brain slices of HIVE mice. Memantine-treated HIVE mice showed significant improvements in synaptic function during frequency facilitation tests and LTP induced by high-frequency stimulation when compared with untreated animals. Immunocytochemical measures of neuronal antigens mirrored the neuronal physiological tests. These results demonstrate that memantine attenuates hippocampal synaptic impairment in murine HIVE and provide a rationale for its use in infected humans who experience cognitive decline.
Figures

References
-
- Anderson ER, Boyle J, Zink WE, Persidsky Y, Gendelman HE, Xiong H (2003) Hippocampal synaptic dysfunction in a murine model of human immunodeficiency virus type 1 encephalitis. Neuroscience 118: 359-369. - PubMed
-
- Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34: 325-337. - PubMed
-
- Barnes CA, Danysz W, Parsons CG (1996) Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur J Neurosci 8: 565-571. - PubMed
-
- Buddle M, Eberhardt E, Ciminello LH, Levin T, Wing R, DiPasquale K, Raley-Susman KM (2003) Microtubule-associated protein 2 (MAP2) associates with the NMDA receptor and is spatially redistributed within rat hippocampal neurons after oxygenglucose deprivation. Brain Res 978: 38-50. - PubMed
-
- Doble A (1995) Excitatory amino acid receptors and neurodegeneration. Therapie 50: 319-337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
