Cotranslational integration and initial sorting at the endoplasmic reticulum translocon of proteins destined for the inner nuclear membrane
- PMID: 15306686
- PMCID: PMC515093
- DOI: 10.1073/pnas.0404934101
Cotranslational integration and initial sorting at the endoplasmic reticulum translocon of proteins destined for the inner nuclear membrane
Abstract
The current diffusion-retention model for protein trafficking to the inner nuclear membrane (INM) proposes that INM proteins diffuse laterally from the membrane of the endoplasmic reticulum into the INM and are then retained in the INM by binding to nuclear proteins or DNA. Because some data indicate that the sorting of baculovirus envelope proteins to the INM is protein-mediated, we have examined the early stages of INM protein integration and sorting by using photocrosslinking. Both viral and host INM-directed proteins were integrated cotranslationally through the endoplasmic reticulum translocon, and their nonrandom photocrosslinking to two translocon proteins, Sec61alpha and translocating chain-associated membrane protein (TRAM), revealed that the first transmembrane sequence (TMS) of each viral and host INM-directed protein occupied a very similar location within the translocon. Because few TMSs of non-INM-directed membrane proteins photocrosslink to TRAM, it seems that the INM-directed TMSs occupy different sites within the translocon than do non-INM-directed TMSs. The distinct proximities of translocon components to INM-directed TMSs strongly suggest that such TMSs are recognized and initially sorted within the translocon. Taken together, these data indicate that membrane protein sorting to the INM is an active process involving specific nonnuclear proteins.
Figures
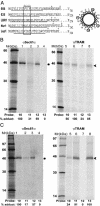
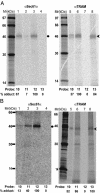
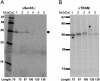

References
-
- Johnson, A. E. & van Waes, M. A. (1999) Annu. Rev. Cell Dev. Biol. 15, 799–842. - PubMed
-
- Schnell, D. J. & Hebert, D. N. (2003) Cell 112, 491–505. - PubMed
-
- Alder, N. N. & Johnson, A. E. (2004) J. Biol. Chem. 279, 22787–22790. - PubMed
-
- Mellman, I. & Warren, G. (2000) Cell 100, 99–112. - PubMed
-
- Barlowe, C. (2003) Trends Cell Biol. 13, 295–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

