Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae
- PMID: 15306853
- PMCID: PMC516630
- DOI: 10.1038/sj.emboj.7600358
Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae
Abstract
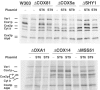
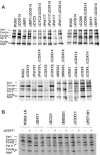
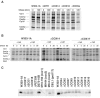
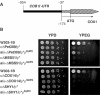
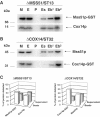
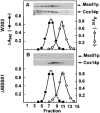
Mutations in SURF1, the human homologue of yeast SHY1, are responsible for Leigh's syndrome, a neuropathy associated with cytochrome oxidase (COX) deficiency. Previous studies of the yeast model of this disease showed that mutant forms of Mss51p, a translational activator of COX1 mRNA, partially rescue the COX deficiency of shy1 mutants by restoring normal synthesis of the mitochondrially encoded Cox1p subunit of COX. Here we present evidence showing that Cox1p synthesis is reduced in most COX mutants but is restored to that of wild type by the same mss51 mutation that suppresses shy1 mutants. An important exception is a null mutation in COX14, which by itself or in combination with other COX mutations does not affect Cox1p synthesis. Cox14p and Mss51p are shown to interact with newly synthesized Cox1p and with each other. We propose that the interaction of Mss51p and Cox14p with Cox1p to form a transient Cox14p-Cox1p-Mss51p complex functions to downregulate Cox1p synthesis. The release of Mss51p from the complex occurs at a downstream step in the assembly pathway, probably catalyzed by Shy1p.
Figures




References
-
- Aldridge P, Hughes KT (2002) Regulation of flagellar assembly. Curr Opin Microbiol 5: 160–165 - PubMed
-
- Barrientos A, Barros MH, Valnot I, Rotig A, Rustin P, Tzagoloff A (2002a) Cytochrome oxidase in health and disease. Gene 286: 53–63 - PubMed
-
- Barros MH, Carlson CG, Glerum DM, Tzagoloff A (2001) Involvement of mitochondrial ferredoxin and Cox15p in hydroxylation of heme O. FEBS Lett 492: 133–138 - PubMed
-
- Barros MH, Tzagoloff A (2002) Regulation of the heme A biosynthetic pathway in Saccharomyces cerevisiae. FEBS Lett 516: 119–123 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

