Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise
- PMID: 15308678
- PMCID: PMC1665266
- DOI: 10.1113/jphysiol.2004.066480
Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise
Abstract
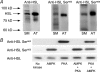
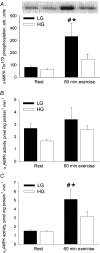
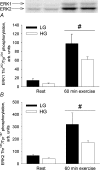
Hormone-sensitive lipase (HSL) catalyses the hydrolysis of myocellular triacylglycerol (MCTG), which is a potential energy source during exercise. Therefore, it is important to elucidate the regulation of HSL activity in human skeletal muscle during exercise. The main purpose of the present study was to investigate the role of 5'AMP-activated protein kinase (AMPK) in the regulation of muscle HSL activity and Ser565 phosphorylation (the presumed AMPK target site) in healthy, moderately trained men during 60 min bicycling (65%). Alpha2AMPK activity during exercise was manipulated by studying subjects with either low (LG) or high (HG) muscle glycogen content. HSL activity was distinguished from the activity of other neutral lipases by immunoinhibition of HSL using an anti-HSL antibody. During exercise a 62% higher (P < 0.01) alpha2AMPK activity in LG than in HG was paralleled by a similar difference (61%, P < 0.01) in HSL Ser565 phosphorylation but without any difference between trials in HSL activity or MCTG hydrolysis. HSL activity was increased (117%, P < 0.05) at 30 min of exercise but not at 60 min of exercise. In both trials, HSL phosphorylation on Ser563 (a presumed PKA target site) was not increased by exercise despite a fourfold increase (P < 0.001) in plasma adrenaline. ERK1/2 phosphorylation was increased by exercise in both trials (P < 0.001) and was higher in LG than in HG both at rest and during exercise (P = 0.06). In conclusion, the present study suggests that AMPK phosphorylates HSL on Ser565 in human skeletal muscle during exercise with reduced muscle glycogen. Apparently, HSL Ser565 phosphorylation by AMPK during exercise had no effect on HSL activity. Alternatively, other factors including ERK may have counterbalanced any effect of AMPK on HSL activity.
Figures

Similar articles
-
Beta-adrenergic stimulation of skeletal muscle HSL can be overridden by AMPK signaling.FASEB J. 2004 Sep;18(12):1445-6. doi: 10.1096/fj.03-1067fje. Epub 2004 Jul 1. FASEB J. 2004. PMID: 15231718 Clinical Trial.
-
Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue.Am J Physiol Endocrinol Metab. 2006 Mar;290(3):E500-8. doi: 10.1152/ajpendo.00361.2005. Epub 2005 Sep 27. Am J Physiol Endocrinol Metab. 2006. PMID: 16188906
-
Effects of plasma adrenaline on hormone-sensitive lipase at rest and during moderate exercise in human skeletal muscle.J Physiol. 2003 Jul 1;550(Pt 1):325-32. doi: 10.1113/jphysiol.2003.043133. Epub 2003 May 2. J Physiol. 2003. PMID: 12730334 Free PMC article.
-
Hormone-sensitive lipase in skeletal muscle: regulatory mechanisms.Acta Physiol Scand. 2003 Aug;178(4):397-403. doi: 10.1046/j.1365-201X.2003.01155.x. Acta Physiol Scand. 2003. PMID: 12864745 Review.
-
Regulation and role of hormone-sensitive lipase in rat skeletal muscle.Proc Nutr Soc. 2004 May;63(2):309-14. doi: 10.1079/PNS2004359. Proc Nutr Soc. 2004. PMID: 15294048 Review.
Cited by
-
A Narrative Review on the Role of AMPK on De Novo Lipogenesis in Non-Alcoholic Fatty Liver Disease: Evidence from Human Studies.Cells. 2021 Jul 19;10(7):1822. doi: 10.3390/cells10071822. Cells. 2021. PMID: 34359991 Free PMC article. Review.
-
Muscle triacylglycerol and hormone-sensitive lipase activity in untrained and trained human muscles.Eur J Appl Physiol. 2006 Jul;97(5):566-72. doi: 10.1007/s00421-006-0220-y. Epub 2006 Jun 10. Eur J Appl Physiol. 2006. PMID: 16767439
-
IL-6 and epinephrine have divergent fiber type effects on intramuscular lipolysis.J Appl Physiol (1985). 2013 Nov;115(10):1457-63. doi: 10.1152/japplphysiol.00558.2013. Epub 2013 Sep 19. J Appl Physiol (1985). 2013. PMID: 24052031 Free PMC article.
-
Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise.J Physiol. 2006 Jul 1;574(Pt 1):17-31. doi: 10.1113/jphysiol.2006.109942. Epub 2006 May 11. J Physiol. 2006. PMID: 16690705 Free PMC article. Review.
-
Hormone-sensitive lipase serine phosphorylation and glycerol exchange across skeletal muscle in lean and obese subjects: effect of beta-adrenergic stimulation.Diabetes. 2008 Jul;57(7):1834-41. doi: 10.2337/db07-0857. Epub 2008 Apr 8. Diabetes. 2008. PMID: 18398140 Free PMC article.
References
-
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. - PubMed
-
- Aronson D, Wojtaszewski JFP, Thorell A, Nygren J, Zangen D, Richter EA, Ljungqvist O, Fielding RA, Goodyear LJ. Extracellular-regulated protein kinase cascades are activated in response to injury in human skeletal muscle. Am J Physiol Cell Physiol. 1998;44:C555–C561. - PubMed
-
- Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Farese RV. Activation of the ERK pathway and atypical protein kinase C Isoforms in exercise- and aminoimidazole-4-carboxamide-1-beta-D-riboside (AICAR)-stimulated glucose transport. J Biol Chem. 2002;277:23554–23562. - PubMed
-
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside – A specific method for activating amp-activated protein-kinase in intact-cells. European J Biochem. 1995;229:558–565. - PubMed
-
- Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

