Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina
- PMID: 15308679
- PMCID: PMC1665265
- DOI: 10.1113/jphysiol.2004.066597
Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina
Abstract
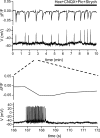
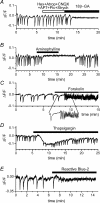
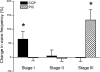
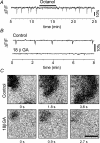
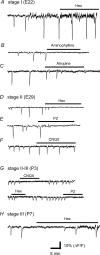
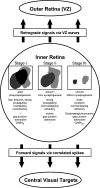
We report here a systematic investigation of the dynamics, regulation and distribution of spontaneous waves in the rabbit retina during the course of wave development prior to eye opening. Three major findings were obtained in this longitudinal study. (1) Spontaneous retinal waves underwent three developmental stages, each of which displayed distinct wave dynamics, pharmacology and mechanism of generation and regulation. Stage I waves emerged prior to synaptogenesis and appeared as frequent, fast propagating waves that did not form spatial boundaries between waves. These waves could be inhibited by blockers of gap junctions and adenosine receptors, but not by nicotinic antagonists. Stage I waves lasted about one day (around embryonic day 22) and then switched rapidly to stage II, resulting in slower and less frequent waves that could be blocked by nicotinic antagonists and had a characteristic postwave refractory period and spatial boundaries between adjacent waves. Immediately after the transition from stage I to stage II, the waves could be reverted back to stage I by blocking nicotinic receptors, indicating the presence of mutually compensatory mechanisms for wave generation. Stage III waves emerged around postnatal day 3-4 (P3-4), and they were mediated by glutamtergic and muscarinic interactions. With age, these waves became weaker, more localized and less frequent. Spontaneous waves were rarely detected after P7. (2) GABA strongly modulated the wave dynamics in a stage- and receptor type-dependent manner. At stage I, endogenous GABAB activation downregulated the waves. The GABAB modulation disappeared during stage II and was replaced by a strong GABA(A/C)-mediated inhibition at stage III. Blocking GABA(A/C) receptors not only dramatically enhanced spontaneous stage III waves, but also induced propagating waves in >P7 retinas that did not show spontaneous waves, indicating a role of GABA inhibition in the disappearance of spontaneous waves. (3) Spontaneous retinal waves were found in both the inner and outer retina at all three stages. The waves in the outer retina (ventricular zone) also showed stage-dependent pharmacology and dynamics. Together, the results revealed a multistaged developmental sequence and stage-dependent dynamics, pharmacology and regulation of spontaneous retinal waves in the mammalian retina. The presence of retinal waves during multiple developmental stages and in multiple retinal layers suggests that the waves are a general developmental phenomenon with diverse functions.
Figures






Similar articles
-
A critical role of the strychnine-sensitive glycinergic system in spontaneous retinal waves of the developing rabbit.J Neurosci. 2001 Jul 15;21(14):5158-68. doi: 10.1523/JNEUROSCI.21-14-05158.2001. J Neurosci. 2001. PMID: 11438591 Free PMC article.
-
GABAergic regulation of spontaneous spike patterns in the developing rabbit retina.Neurosci Lett. 2015 Jul 23;600:137-42. doi: 10.1016/j.neulet.2015.06.003. Epub 2015 Jun 6. Neurosci Lett. 2015. PMID: 26054939
-
Coordinated transitions in neurotransmitter systems for the initiation and propagation of spontaneous retinal waves.J Neurosci. 2000 Sep 1;20(17):6570-7. doi: 10.1523/JNEUROSCI.20-17-06570.2000. J Neurosci. 2000. PMID: 10964962 Free PMC article.
-
The role of early neural activity in the maturation of turtle retinal function.J Anat. 2001 Oct;199(Pt 4):375-83. doi: 10.1046/j.1469-7580.2001.19940375.x. J Anat. 2001. PMID: 11693298 Free PMC article. Review.
-
Retinal waves and visual system development.Annu Rev Neurosci. 1999;22:29-47. doi: 10.1146/annurev.neuro.22.1.29. Annu Rev Neurosci. 1999. PMID: 10202531 Review.
Cited by
-
Triphasic spike-timing-dependent plasticity organizes networks to produce robust sequences of neural activity.Front Comput Neurosci. 2012 Nov 12;6:88. doi: 10.3389/fncom.2012.00088. eCollection 2012. Front Comput Neurosci. 2012. PMID: 23162457 Free PMC article.
-
Disruption and recovery of patterned retinal activity in the absence of acetylcholine.J Neurosci. 2005 Oct 12;25(41):9347-57. doi: 10.1523/JNEUROSCI.1800-05.2005. J Neurosci. 2005. PMID: 16221843 Free PMC article.
-
The role of neuronal connexins 36 and 45 in shaping spontaneous firing patterns in the developing retina.J Neurosci. 2011 Jul 6;31(27):9998-10008. doi: 10.1523/JNEUROSCI.5640-10.2011. J Neurosci. 2011. PMID: 21734291 Free PMC article.
-
Following the ontogeny of retinal waves: pan-retinal recordings of population dynamics in the neonatal mouse.J Physiol. 2014 Apr 1;592(7):1545-63. doi: 10.1113/jphysiol.2013.262840. Epub 2013 Dec 23. J Physiol. 2014. PMID: 24366261 Free PMC article.
-
Muscarinic acetylcholine receptor localization and activation effects on ganglion response properties.Invest Ophthalmol Vis Sci. 2010 May;51(5):2778-89. doi: 10.1167/iovs.09-4771. Epub 2009 Dec 30. Invest Ophthalmol Vis Sci. 2010. PMID: 20042645 Free PMC article.
References
-
- Ames A, Nesbett FB. In vitro retina as an experimental model of the central nervous system. J Neurochem. 1981;37:867–877. - PubMed
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. - PMC - PubMed
-
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
-
- Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295:862–869. - PubMed
-
- Catsicas M, Bonness V, Becker D, Mobbs P. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol. 1998;8:283–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

