Phospholipid scramblase 1 potentiates the antiviral activity of interferon
- PMID: 15308695
- PMCID: PMC506946
- DOI: 10.1128/JVI.78.17.8983-8993.2004
Phospholipid scramblase 1 potentiates the antiviral activity of interferon
Abstract
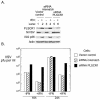
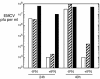
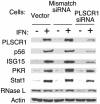
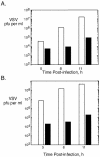
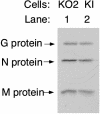
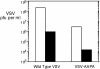
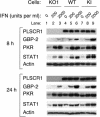
Phospholipid scramblase 1 (PLSCR1) is an interferon (IFN)- and growth factor-inducible, calcium-binding protein that either inserts into the plasma membrane or binds DNA in the nucleus depending on its state of palmyitoylation. In certain hematopoietic cells, PLSCR1 is required for normal maturation and terminal differentiation from progenitor cells as regulated by select growth factors, where it promotes recruitment and activation of Src kinases. PLSCR1 is a substrate of Src (and Abl) kinases, and transcription of the PLSCR1 gene is regulated by the same growth factor receptor pathways in which PLSCR1 potentiates afferent signaling. The marked transcriptional upregulation of PLSCR1 by IFNs led us to explore whether PLSCR1 plays an analogous role in cellular responses to IFN, with specific focus on antiviral activities. Accordingly, human cells in which PLSCR1 expression was decreased with short interfering RNA were rendered relatively insensitive to the antiviral activity of IFNs, resulting in higher titers of vesicular stomatitis virus (VSV) and encephalomyocarditis virus. Similarly, VSV replicated to higher titers in mouse PLSCR1(-/-) embryonic fibroblasts than in identical cells transduced to express PLSCR1. PLSCR1 inhibited accumulation of primary VSV transcripts, similar to the effects of IFN against VSV. The antiviral effect of PLSCR1 correlated with increased expression of a subset of IFN-stimulated genes (ISGs), including ISG15, ISG54, p56, and guanylate binding proteins. Our results suggest that PLSCR1, which is itself an ISG-encoded protein, provides a mechanism for amplifying and enhancing the IFN response through increased expression of a select subset of potent antiviral genes.
Figures


Similar articles
-
Phospholipid scramblase 1 binds to the promoter region of the inositol 1,4,5-triphosphate receptor type 1 gene to enhance its expression.J Biol Chem. 2005 Oct 14;280(41):35062-8. doi: 10.1074/jbc.M504821200. Epub 2005 Aug 9. J Biol Chem. 2005. PMID: 16091359
-
c-Abl tyrosine kinase binds and phosphorylates phospholipid scramblase 1.J Biol Chem. 2001 Aug 3;276(31):28984-90. doi: 10.1074/jbc.M102505200. Epub 2001 Jun 4. J Biol Chem. 2001. PMID: 11390389
-
Inhibition of Hepatitis B virus replication by phospholipid scramblase 1 in vitro and in vivo.Antiviral Res. 2012 Apr;94(1):9-17. doi: 10.1016/j.antiviral.2012.01.010. Epub 2012 Feb 9. Antiviral Res. 2012. PMID: 22342889
-
Phospholipid scramblase 1: a frontline defense against viral infections.Front Cell Infect Microbiol. 2025 Apr 3;15:1573373. doi: 10.3389/fcimb.2025.1573373. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40248364 Free PMC article. Review.
-
Systems biology of interferon responses.J Interferon Cytokine Res. 2011 Jan;31(1):5-11. doi: 10.1089/jir.2010.0126. J Interferon Cytokine Res. 2011. PMID: 21226606 Review.
Cited by
-
A genome-wide arrayed CRISPR screen identifies PLSCR1 as an intrinsic barrier to SARS-CoV-2 entry that recent virus variants have evolved to resist.PLoS Biol. 2024 Sep 24;22(9):e3002767. doi: 10.1371/journal.pbio.3002767. eCollection 2024 Sep. PLoS Biol. 2024. PMID: 39316623 Free PMC article.
-
Identification of phospholipid scramblase 1 as a biomarker and determination of its prognostic value for colorectal cancer.Mol Med. 2011 Jan-Feb;17(1-2):41-7. doi: 10.2119/molmed.2010.00115. Epub 2010 Oct 5. Mol Med. 2011. PMID: 20927484 Free PMC article.
-
ILDR1 promotes influenza A virus replication through binding to PLSCR1.Sci Rep. 2022 May 20;12(1):8515. doi: 10.1038/s41598-022-12598-3. Sci Rep. 2022. PMID: 35595813 Free PMC article.
-
Hepatitis C virus infection protein network.Mol Syst Biol. 2008;4:230. doi: 10.1038/msb.2008.66. Epub 2008 Nov 4. Mol Syst Biol. 2008. PMID: 18985028 Free PMC article.
-
Genetic Associations with Coronavirus Susceptibility and Disease Severity.Adv Exp Med Biol. 2023;1412:119-140. doi: 10.1007/978-3-031-28012-2_6. Adv Exp Med Biol. 2023. PMID: 37378764 Review.
References
-
- Anderson, S. L., J. M. Carton, J. Lou, L. Xing, and B. Y. Rubin. 1999. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology 256:8-14. - PubMed
-
- Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. - PubMed
-
- Barenholz, Y., R. Pal, and R. R. Wagner. 1993. Metabolic labeling of viral membrane lipids by fluorescent fatty acids: studying virus fusion with target membranes. Methods Enzymol. 220:288-312. - PubMed
-
- Baumal, R., J. Law, R. N. Buick, H. Kahn, H. Yeger, K. Sheldon, T. Colgan, and A. Marks. 1986. Monoclonal antibodies to an epithelial ovarian adenocarcinoma: distinctive reactivity with xenografts of the original tumor and a cultured cell line. Cancer Res. 46:3994-4000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

