Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A*0201 transgenic mice
- PMID: 15308705
- PMCID: PMC506963
- DOI: 10.1128/JVI.78.17.9093-9104.2004
Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A*0201 transgenic mice
Abstract
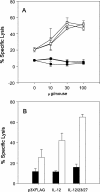
Searching the sequence databases has revealed two novel cytokines: interleukin-23 (IL-23) and IL-27. These cytokines are quite similar to, but clearly distinct from IL-12 in their structures and T-cell stimulatory fashions. In contrast to IL-12, however, little is known about the roles of IL-23 and IL-27 in the immune regulation. Previously, we evaluated the prime-boost immunization consisting of priming and the first boosting with the hepatitis C virus (HCV)-core expression plasmid, followed by a second boosting with recombinant adenovirus expressing HCV core for induction of HCV core-specific cytotoxic T lymphocytes (CTLs) in BALB/c mice. The present study demonstrates that HCV-specific CTL induction was greatly enhanced by coinoculation of an IL-12 expression plasmid in the prime-boost immunization, indicating the potent adjuvant activity of IL-12. We investigated whether similar adjuvant effects could be exerted by either IL-23 or IL-27 in a prime-boost immunization with HLA-A*0201 transgenic mice. Coadministration of either an IL-23 or an IL-27 expression plasmid, as well as an IL-12 expression plasmid, in a prime-boost immunization enhanced induction of HCV-specific CTLs and led to dramatic increases in the numbers of gamma interferon (IFN-gamma)-producing, HCV-specific CD8+ cells. Further, preinjections of IL-12, IL-23, or IL-27 expression plasmids before immunization resulted in great increases in the number of IFN-gamma-producing, HCV-specific CD8+ cells in response to immunization with recombinant adenovirus. These data revealed that both IL-23 and IL-27, as well as IL-12, are potent adjuvants for epitope-specific CTL induction. The two novel cytokines might offer new prophylactic and therapeutic strategies against infectious pathogens such as HCV.
Figures



Similar articles
-
Enhanced induction of hepatitis C virus-specific cytotoxic T lymphocytes and protective efficacy in mice by DNA vaccination followed by adenovirus boosting in combination with the interleukin-12 expression plasmid.Vaccine. 2003 Apr 2;21(15):1629-39. doi: 10.1016/s0264-410x(02)00704-1. Vaccine. 2003. PMID: 12639484
-
DNA immunization with hepatitis C virus (HCV) polycistronic genes or immunization by HCV DNA priming-recombinant canarypox virus boosting induces immune responses and protection from recombinant HCV-vaccinia virus infection in HLA-A2.1-transgenic mice.J Virol. 2003 Jan;77(1):382-90. doi: 10.1128/jvi.77.1.382-390.2003. J Virol. 2003. PMID: 12477843 Free PMC article.
-
DNA fusion vaccines induce epitope-specific cytotoxic CD8(+) T cells against human leukemia-associated minor histocompatibility antigens.Cancer Res. 2006 May 15;66(10):5436-42. doi: 10.1158/0008-5472.CAN-05-3130. Cancer Res. 2006. PMID: 16707472
-
Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27.Arch Immunol Ther Exp (Warsz). 2006 Jan-Feb;54(1):15-24. doi: 10.1007/s00005-006-0002-6. Epub 2006 Jan 23. Arch Immunol Ther Exp (Warsz). 2006. PMID: 16642253 Review.
-
A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes.J Biomed Biotechnol. 2010;2010:605483. doi: 10.1155/2010/605483. Epub 2010 Apr 29. J Biomed Biotechnol. 2010. PMID: 20454646 Free PMC article. Review.
Cited by
-
Expression of IL-27 by tumor cells in invasive cutaneous and metastatic melanomas [corrected].PLoS One. 2013 Oct 10;8(10):e75694. doi: 10.1371/journal.pone.0075694. eCollection 2013. PLoS One. 2013. PMID: 24130734 Free PMC article.
-
Inteleukin-23 promotes interferon-α responsiveness in hepatitis C virus/HIV-coinfected patients.AIDS Res Hum Retroviruses. 2014 Aug;30(8):775-82. doi: 10.1089/aid.2014.0003. Epub 2014 Jun 30. AIDS Res Hum Retroviruses. 2014. PMID: 24856902 Free PMC article.
-
Highly Pathogenic Avian Influenza (HPAI) H5 Clade 2.3.4.4b Virus Infection in Birds and Mammals.Animals (Basel). 2024 May 2;14(9):1372. doi: 10.3390/ani14091372. Animals (Basel). 2024. PMID: 38731377 Free PMC article. Review.
-
Enhanced cell immune responses to hepatitis C virus core by novel heterologous DNA prime/lambda nanoparticles boost in mice.Virus Genes. 2014 Aug;49(1):11-21. doi: 10.1007/s11262-014-1070-z. Epub 2014 Apr 22. Virus Genes. 2014. PMID: 24752903
-
Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27.Clin Dev Immunol. 2010;2010:832454. doi: 10.1155/2010/832454. Epub 2010 Sep 14. Clin Dev Immunol. 2010. PMID: 20885915 Free PMC article. Review.
References
-
- Alter, M. J., H. S. Margolis, K. Krawczynski, F. N. Judson, A. Mares, W. J. Alexander, P. Y. Hu, J. K. Miller, M. A. Gerber, and R. E. Sampliner. 1992. The natural history of community-acquired hepatitis C in the United States: the Sentinel Counties chronic non-A, non-B hepatitis study team. N. Engl. J. Med. 327:1899-1905. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Bain, C., A. Fatmi, F. Zoulin, J.-P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120:512-524. - PubMed
-
- Battegay, M., J. Fikes, A. M. Di Bisceglie, P. A. Wentworth, A. Sette, E. Celis, W.-M. Ching, A. Grakoui, C. M. Rice, K. Kurokohchi, J. A. Berzofsky, J. H. Hoofnagle, S. M. Feinstone, and T. Akatsuka. 1995. Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus-encoded peptides binding to HLA-A2.1 molecules. J. Virol. 69:2462-2470. - PMC - PubMed
-
- Belladonna, M. L., J.-C. Renauld, R. Bianchi, C. Vacca, F. Fallarino, C. Orabona, M. C. Fioretti, U. Grohmann, and P. Puccetti. 2001. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 168:5448-5454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

