Host cell gene expression during human immunodeficiency virus type 1 latency and reactivation and effects of targeting genes that are differentially expressed in viral latency
- PMID: 15308739
- PMCID: PMC506933
- DOI: 10.1128/JVI.78.17.9458-9473.2004
Host cell gene expression during human immunodeficiency virus type 1 latency and reactivation and effects of targeting genes that are differentially expressed in viral latency
Abstract
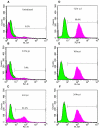
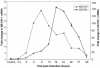
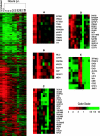
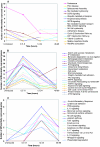
The existence of reservoirs of cells latently infected with human immunodeficiency virus (HIV) is a major obstacle to the elimination of HIV infection. We studied the changes in cellular gene expression that accompany the reactivation and completion of the lytic viral cycle in cell lines chronically infected with HIV-1. We found that several genes exhibited altered expression in the chronically infected cells compared to the uninfected parental cells prior to induction into lytic replication. A number of gene classes showed increased expression in the chronically infected cells, notably including genes encoding proteasomes, histone deacetylases, and many transcription factors. Following induction of the lytic replication cycle, we observed ordered, time-dependent changes in the cellular gene expression pattern. Approximately 1,740 genes, many of which fall into 385 known pathways, were differentially expressed (P < 0.001), indicating that completion of the HIV replication cycle is associated with distinct, temporally ordered changes in host cell gene expression. Maximum changes were observed in the early and intermediate phases of the lytic replication cycle. Since the changes in gene expression in chronically infected cells suggested that cells latently infected with HIV have a different gene expression profile than corresponding uninfected cells, we studied the expression profiles of three different chronically infected cell lines to determine whether they showed similar changes in common cellular genes and pathways. Thirty-two genes showed significant differential expression in all cell lines studied compared to their uninfected parental cell lines. Notable among them were cdc42 and lyn, which were downregulated and are required for HIV Nef binding and viral replication. Other genes previously unrelated to HIV latency or pathogenesis were also differentially expressed. To determine the effects of targeting products of the genes that were differentially expressed in latently infected cells, we treated the latently infected cells with a proteasome inhibitor, clastolactacystin-beta-lactone (CLBL), and an Egr1 activator, resveratrol. We found that treatment with CLBL and resveratrol stimulated lytic viral replication, suggesting that treatment of cells with agents that target cellular genes differentially expressed in latently infected cells can stimulate lytic replication. These findings may offer new insights into the interaction of the latently infected host cell and HIV and suggest therapeutic approaches for inhibiting HIV infection and for manipulating cells latently infected with HIV so as to trigger lytic replication.
Figures

References
-
- Adams, J. 2003. Potential for proteasome inhibition in the treatment of cancer. Drug Discov. Today 8:307-315. - PubMed
-
- Adams, J. 2001. Proteasome inhibition in cancer: development of PS-341. Semin. Oncol. 28:613-619. - PubMed
-
- Adams, J. 2003. The proteasome: structure, function, and role in the cell. Cancer Treat. Rev. 29(Suppl. 1):3-9. - PubMed
-
- Ashburner, M., C. A. Ball, J. A. Blake, D. Botstein, H. Butler, J. M. Cherry, A. P. Davis, K. Dolinski, S. S. Dwight, J. T. Eppig, M. A. Harris, D. P. Hill, L. Issel-Tarver, A. Kasarskis, S. Lewis, J. C. Matese, J. E. Richardson, M. Ringwald, G. M. Rubin, and G. Sherlock. 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25:25-29. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

