Pathogenesis of acute viral disease induced in fish by carp interstitial nephritis and gill necrosis virus
- PMID: 15308746
- PMCID: PMC506927
- DOI: 10.1128/JVI.78.17.9544-9551.2004
Pathogenesis of acute viral disease induced in fish by carp interstitial nephritis and gill necrosis virus
Abstract
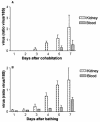
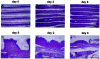
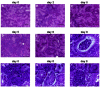
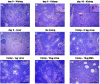
A lethal disease of koi and common carp (species Cyprinus carpio) has afflicted many fish farms worldwide since 1998, causing severe financial losses. Morbidity and mortality are restricted to common carp and koi and appear in spring and autumn, when water temperatures are 18 to 28 degrees C. We have isolated the virus causing the disease from sick fish, propagated it in koi fin cell culture, and shown that virus from a single clone causes lethal disease in carp and koi upon infection. Intraperitoneal virus injection or bathing the fish in virus-containing water kills 85 to 100% of the fish within 7 to 21 days. This virus is similar to the previously reported koi herpesvirus; however, it has characteristics inconsistent with the herpesvirus family, and thus we have called it carp interstitial nephritis and gill necrosis virus. We examined the pathobiology of this disease in carp by using immunohistochemistry and PCR. We found large amounts of the virus in the kidneys of sick fish and smaller amounts in liver and brain. A rapid increase in the viral load in the kidneys was detected by using both immunofluorescence and semiquantitative PCR. Histological analyses of fish at various times after infection revealed signs of interstitial nephritis as early as 2 days postinfection, which increased in severity up to 10 days postinfection. There was severe gill disease evidenced by loss of villi with accompanying inflammation in the gill rakers. Minimal focal inflammation was noted in livers and brains. This report describes the etiology and pathology of a recently described viral agent in fish.
Figures



References
-
- Body, A., F. Lieffrig, G. Charlier, and A. Collard. 2000. Isolation of virus-like particles from Koi (Cyprinus carpio) suffering gill necrosis. Bull. Eur. Assoc. Fish Pathol. 20:87-88.
-
- Brudeseth, B. E., J. Castric, and O. Evensen. 2002. Studies on pathogenesis following single and double infection with viral hemorrhagic septicemia virus and infectious hematopoietic necrosis virus in rainbow trout (Oncorhynchus mykiss). Vet. Pathol. 39:180-189. - PubMed
-
- Davison, A. J. 2002. Evolution of the herpes viruses. Vet. Microbiol. 86:69-88. - PubMed
-
- Gilad, O., S. Yun, K. B. Andree, M. A. Adkison, A. Zlotkin, H. Bercovier, A. Eldar, and R. P. Hedrick. 2002. Initial characteristics of koi herpesvirus and development of a polymerase chain reaction assay to detect the virus in koi, Cyprinus carpio koi. Dis. Aquat. Organ. 48:101-108. - PubMed
-
- Gilad, O., S. Yun, M. A. Adkison, K. Way, N. H. Willits, H. Bercovier, and R. P. Hedrick. 2003. Molecular comparison of isolates of an emerging fish pathogen, koi herpesvirus, and the effect of water temperature on mortality of experimentally infected koi. J. Gen. Virol. 84:2661-2668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

