Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis
- PMID: 15308753
- PMCID: PMC520945
- DOI: 10.1105/tpc.104.022608
Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis
Abstract
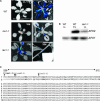
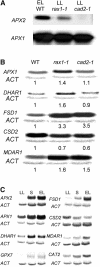
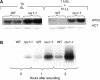
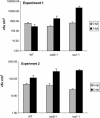
The mutant regulator of APX2 1-1 (rax1-1) was identified in Arabidopsis thaliana that constitutively expressed normally photooxidative stress-inducible ASCORBATE PEROXIDASE2 (APX2) and had >/=50% lowered foliar glutathione levels. Mapping revealed that rax1-1 is an allele of gamma-GLUTAMYLCYSTEINE SYNTHETASE 1 (GSH1), which encodes chloroplastic gamma-glutamylcysteine synthetase, the controlling step of glutathione biosynthesis. By comparison of rax1-1 with the GSH1 mutant cadmium hypersensitive 2, the expression of 32 stress-responsive genes was shown to be responsive to changed glutathione metabolism. Under photo-oxidative stress conditions, the expression of a wider set of defense-related genes was altered in the mutants. In wild-type plants, glutathione metabolism may play a key role in determining the degree of expression of defense genes controlled by several signaling pathways both before and during stress. This control may reflect the physiological state of the plant at the time of the onset of an environmental challenge and suggests that changes in glutathione metabolism may be one means of integrating the function of several signaling pathways.
Figures


References
-
- Asada, K. (1999). The water-water cycle in chloroplasts: Scavenging of active oxygen species and dissipation of excess photons. Annu. Rev. Plant Physiol. Mol. Biol. 50, 601–639. - PubMed
-
- Ball, L. (2001). Identification of Arabidopsis Mutants with Altered ASCORBATE PEROXIDASE II Gene Expression. PhD dissertation (Norwich, UK: University of East Anglia).
-
- Berger, S., Mitchell-Olds, T., and Stotz, H.U. (2002). Local and differential control of vegetative storage protein expression in response to herbivore damage in Arabidopsis thaliana. Physiol. Plant 114, 85–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

